��Ŀ����
14��ʵ������������NaOH��Һ�����м���Һ���ʵ���Ũ��δ֪������Һ���ʵ���Ũ��Ϊ3mol/L�����߾�δ���ʣ���300mL����Һ��100mL����Һ��ϣ���������仯���õ�400mL����Һ������Һ�м���600mL 1mol/L���������ԣ���1�������Һ���ʵ���Ũ�ȣ�
��2�������Һ���ʵ���Ũ�ȣ�
��3��������Һ�ܶ�Ϊ1.2g/mL����ô����Һ���ܼ����������������Ķ��ٱ���
���� ����Һ�����ᷢ����Ӧ��NaOH+HCl=NaCl+H2O������n=cV��������HCl���ʵ������ɷ���ʽ��֪����Һ��NaOH���ʵ������ٸ���c=$\frac{n}{V}$�������Һ���ʵ���Ũ�ȣ�
�ס�����Һ��NaOH���ʵ���֮�ʹ��ڱ���Һ��NaOH���ʵ������ݴ˼������Һ���ʵ���Ũ�ȣ�
����m=��V�������Һ����������m=nM�������Һ��NaOH����������Һ��ˮ������=��Һ������-NaOH��������
��� �⣺����Һ�����ᷢ����Ӧ��NaOH+HCl=NaCl+H2O������HCl���ʵ���=0.6L��1mol/L=0.6mol����n��NaOH��=n��HCl��=0.6mol���ʱ���Һ���ʵ���Ũ��$\frac{0.6mol}{0.4L}$=1.5mol/L��
�����Һ���ʵ���Ũ��Ϊcmol/L����0.3L��cmol/L+0.1L��3mol/L=0.6mol�����c=1��������ҺŨ��Ϊ1mol/L��
����Һ����=400mL��1.2g/mL=480g��NaOH������=0.6mol��40g/mol=24g������Һ��ˮ������=480g-24g=456g������Һ���ܼ�����������������$\frac{456g}{24g}$=19����
�𣺣�1������Һ���ʵ���Ũ��Ϊ1mol/L��
��2������Һ���ʵ���Ũ��Ϊ1.5mol/L��
��3������Һ���ܼ�����������������19����
���� ���⿼�����ʵ���Ũ���йؼ��㣬�Ƚϻ�����ע��Ի���֪ʶ���������գ�

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�| A�� | ϡ���� | B�� | Fe��NO3��3��Һ | C�� | CuSO4��Һ | D�� | AgNO3��Һ |
| A�� | ���³�ѹ�º���NA��ԭ�ӵĺ������ԼΪ22.4L | |
| B�� | ���³�ѹ�£�O2��O3�Ļ����16g��Լ����6.02��1023����ԭ�� | |
| C�� | ��0�棬101KPaʱ��22.4L�����к���NA����ԭ�� | |
| D�� | ��״���£�33.6LH2O����1.5NA��H2O���� |
| A�� | ��ɫ���������Һ������SO2����Һ��ɫ��ȥ��˵��SO2����Ư���� | |
| B�� | ��ij��Һ�еμ������ữ���Ȼ�����Һ������ɫ������˵������Һ��һ������SO32- | |
| C�� | ͭ�����ĺϽ���1L 1mol/L������ǡ�÷�Ӧ������Fe2+����δ����ԭ����������ʵ���Ϊ0.75mol | |
| D�� | ����������Ӧ�����Ȼ��������Բ����ø�ƿʢ��Һ�� |
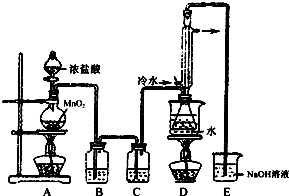 S2Cl2�ǹ�ҵ�ϳ��õ�����ʵ�����Ʊ�S2Cl2�ķ�Ӧԭ�������֣�
S2Cl2�ǹ�ҵ�ϳ��õ�����ʵ�����Ʊ�S2Cl2�ķ�Ӧԭ�������֣���CS2+3Cl2$\frac{\underline{\;95��100��\;}}{\;}$CCl4+S2Cl2��
��2S+Cl2$\frac{\underline{\;111��140��\;}}{\;}$S2Cl2��
��֪��S2Cl2����ˮ��Ӧ��S2Cl2+Cl2$\stackrel{��}{?}$2SCl2��
�����Ǽ������ʵ��۷е�ͷ�Ӧװ��ͼ
| ���� | �е�/�� | �۵�/�� |
| S | 445 | 113 |
| CS2 | 47 | -109 |
| CCl4 | 77 | -23 |
| S2Cl2 | 137 | -77 |
��2����װ��C�����ɸ���ܣ���װ��C�п�ѡ�õĹ����Լ�����ˮ�Ȼ��ƻ����������ף�
��3��Dװ���������ܵ�������������������������Ӧ������Dװ����ƿ�ڵĻ�����з��������ķ���������
��4��S2Cl2������ˮ��Ӧ�л�ɫ�������ɣ���������ɫ������ʹƷ����Һ��ɫ����÷�Ӧ�Ļ�ѧ����ʽΪ2S2Cl2+2H2O�T3S��+SO2��+4HCl����
��5��Ϊ������ƵõIJ�ƷS2Cl2�Ĵ��ȣ��ؼ��IJ����ǿ��ƺ��¶ȺͿ���Ũ����ĵ��ٲ���̫�죮
��6��ͼ��β��װ�ò������ƣ����ڵ���������D��E֮�����Ӹ���װ�ã�ͬʱβ������Ҫ��������
| A�� | 0.7L | B�� | 1.0L | C�� | 1.6L | D�� | 2.5L |
��ͬλ�� ��ͬ�������� ��ͬ���칹�� ��ͬϵ�� ��ͬ������
| ���� ���� | ���� ����� | ������ ʮ���� | �ȷ��� ���ȼ��� | ��� 뮡�� | 2--�������� 2��3--�������� |
| � ��ϵ |
A��CH3CH2CH2CHO��
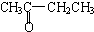 B��
B��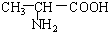 ��
��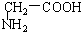 C��
C��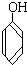 ��
��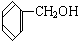
D��CH3CH2C��CH��CH2�TCH-CH=CH2E��
 ��
��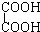 F����ϩ�ͻ�����
F����ϩ�ͻ������������������еĺ����������ǣ�д���ƣ�ȩ�����ʻ����Ȼ������ݹ����ŵ��ص�ɽ�C���������ﻮ��Ϊ����ʹ��࣮
������A�������������ij��ϩ�������Ը��������Һ�������ɵģ���õ�ϩ���Ľṹ��ʽΪCH3CH2CH2CH=C��CH3��CH2CH3��