��Ŀ����
���з�Ӧ�����ӷ���ʽ��ȷ����
| A��ͭ����Ũ���Cu + 4H+ + 2NO3-= Cu2+ + 2NO2��+ 2H2O |
| B���Ȼ�����Һ�������ˮ��Ӧ��Al3++4NH3��H2O= [Al(OH)4]-+4NH4+ |
| C��������ˮ��Ӧ��Cl2+H2O��2H++Cl-+ClO- |
| D������SO2ͨ��Ca(ClO)2��Һ�У�SO2+H2O+Ca2++2ClO��=CaSO3��+2HClO |
A
�������������A.ԭ������д��������ȡ��B.һˮ�ϰ���������ʣ������ܽ��������������ӷ���ʽӦ��Ϊ��Al3++3NH3��H2O= Al(OH)3��+3NH4+.����C.HClO�����ᣬӦ��д��ѧʽ�����ӷ���ʽΪCl2+H2O��H++Cl-+HClO������D.HClO�������ԣ����CaSO3����ΪCaSO4�����ӷ���ʽһ��д����SO2+H2O+Ca2++ClO��=CaSO4��+2H++Cl-������
���㣺�������ӷ���ʽ�������жϵ�֪ʶ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�������ӷ���ʽ��ȷ����
| A����������Һ��ͨ������CO2��2C6H5O����CO2��H2O��2C6H5OH��CO32�� |
B����ȩ��Һ�м���������������Һ�����ȣ�HCHO��2[Ag(NH3)2]����2OH�� HCOO����NH4����2Ag����3NH3��H2O HCOO����NH4����2Ag����3NH3��H2O |
C����ȩ�����������ͭ����Һ��Ϻ���������ڣ�CH3CHO��2Cu(OH)2��OH�� Cu2O����CH3COO����3H2O Cu2O����CH3COO����3H2O |
| D����С�մ���Һ�м����� CO32����2CH3COOH =CO2����H2O��2CH3COO�� |
�������ӷ���ʽ��д��ȷ����
| A������Ͷ�뵽NaOH��Һ�У�2Al+2OH����2AlO2��+H2�� |
| B��AlCl3��Һ�м��������İ�ˮ��Al3++ 3OH����Al(OH)3�� |
| C�����Ȼ�����Һ�м������ۣ�Fe3����Fe��2Fe2�� |
| D��FeCl2��Һ��Cl2��Ӧ��2Fe2++Cl2��2Fe3++2Cl�� |
ij��Na+����Һ�п��ܺ���NH4+��Fe3+��Br����CO32����I����SO32����ȡ�����μ�������ˮ�������ݲ�������Һ�ʳ�ɫ�����ɫ��Һ�м�BaCl2��Һ����۾�������Ϊȷ������Һ����ɣ�������е�ʵ����
| A��ȡ�����μ����軯����Һ |
| B��ȡ��������ˮ��CCl4���� |
| C��ȡ����������������ȣ�ʪ���Ʒ����ֽ�������� |
| D��ȡ��������������ʯ�Ҽ��ȣ�ʪ��ĺ�ɫʯ����ֽ�������� |
�������ӷ���ʽ��ȷ����
A��HCO3��ˮ������ӷ���ʽ��HCO3����H2O CO32����H3O�� CO32����H3O�� |
| B��Ư����Һ�ڿ�����ʧЧ��ClO����CO2��H2O��HClO��HCO3�� |
| C��������SO2ͨ��NaOH��Һ�У�SO2��+2OH����SO32��+H2O |
D������������ͭ����ȩ��Ӧ��CH3CHO��2Cu(OH)2��OH��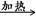 CH3COO����Cu2O����3H2O CH3COO����Cu2O����3H2O |
���з�Ӧ�����ӷ���ʽ��д��ȷ����
| A������ʯ������ķ�Ӧ�� CO32��+ 2H+ = CO2��+ H2O |
| B�����Ȼ�����Һ�м��������ˮ�� Al3+ + 4NH3?H2O = AlO2��+ 2H2O + 4NH4+ |
| C������������������Һ��Ӧ��Cl2 + OH�� =" Cl��" + HClO |
D����ⱥ��ʳ��ˮ��2Cl��+ 2H2O  2OH�� + Cl2��+ H2�� 2OH�� + Cl2��+ H2�� |
��������Һ�У���������������Һ���ܴ����������
| A��Cu2+��NH4+��Br-��OH- | B��K+��Ba2+��OH-��SO42- |
| C��Ag+��NO3-��Cl-��K+ | D��Cl-��SO42-��K+��Na+ |
���и�����������Һ���ܴ����������
| A��K+��Na+��SO42-��CO32- | B��NH4+��Na+��SO42-��OH- |
| C��H+��K+��HCO3-��Cl- | D��Al3+��K+��OH-��NO3- |
���и���������ָ����Һ�У�һ���д����������
| A��pH��0����Һ�У�Na����[Al(OH)4]����K+��NH4+ |
| B����ˮ�������c(H��)��10��12mol/L����Һ�У�C1-��HCO3-��NH4+��SO32- |
| C�������������H2����Һ�У�Mg2+��NH4+��C1-��SO42- |
| D���μ�ʯ����Һ����ɫ����Һ��K+��Ba2+��NO3-��OH- |