��Ŀ����
����ֵ�����ܶ࣬����һ����Ԫ�����⣬�����н϶��Cr(������Ni(������Mo(�⣩��������Si(�裩��C(̼��������S(��P(�ף���
��1������Ԫ�������ڵ��������ҵ�һ�����ܴӴ�С���е���_______
��2��Mo���⣩���ڵ������ڣ�����Cr������Ԫ��λ��ͬһ�壬���̬Mo���⣩ԭ�ӵ���Χ���ӣ��۵��ӣ��Ų�ʽ��_______��
��3��CH4�ķе��SiH4�ͣ�ԭ����_______��
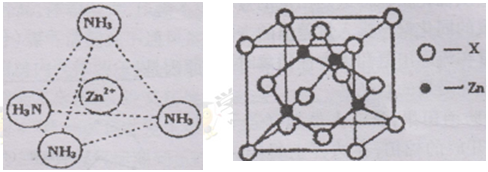
��4����Ԫ�����γɶ�������������[Ni(CN)4]2���в����е���____����ѡ���ţ���
��5��̼Ԫ���ж��ֵ��ʣ�����C60�����ж��ص����νṹ��C60������ԭ�ӹ���ӻ�������_______��

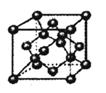
��6��̼����һ�ֵ���ʯī�ʲ�״�ṹ����һ̼þ���Ͳ��Ͼ�����ʯī̼ԭ�Ӳ�����þԭ�Ӳ㣬���㸩��ͼ��

�ò��ϵĻ�ѧʽΪ_______��
��1������Ԫ�������ڵ��������ҵ�һ�����ܴӴ�С���е���_______
��2��Mo���⣩���ڵ������ڣ�����Cr������Ԫ��λ��ͬһ�壬���̬Mo���⣩ԭ�ӵ���Χ���ӣ��۵��ӣ��Ų�ʽ��_______��
��3��CH4�ķе��SiH4�ͣ�ԭ����_______��
��4����Ԫ�����γɶ�������������[Ni(CN)4]2���в����е���____����ѡ���ţ���
| A�����Ӽ� | B����λ�� | C���Ҽ� | D���м�E����� |

��6��̼����һ�ֵ���ʯī�ʲ�״�ṹ����һ̼þ���Ͳ��Ͼ�����ʯī̼ԭ�Ӳ�����þԭ�Ӳ㣬���㸩��ͼ��

�ò��ϵĻ�ѧʽΪ_______��
��13�֣�
��1��P��S��Si��2�֣�
��2��4d55s1��2�֣�
��3��CH4��SiH4��ɡ��ṹ���ƣ�CH4����Է���������С�����Ӽ䷶�»���Ҳ��С������е�ϵͣ�2�֣�
��4��A E��2�֣�
��5��sp2��2�֣�
��6��MgC2��3�֣�
��1��P��S��Si��2�֣�
��2��4d55s1��2�֣�
��3��CH4��SiH4��ɡ��ṹ���ƣ�CH4����Է���������С�����Ӽ䷶�»���Ҳ��С������е�ϵͣ�2�֣�
��4��A E��2�֣�
��5��sp2��2�֣�
��6��MgC2��3�֣�
�����������1��Si��P��S�ǵ������ڴ����ҵ�����Ԫ�أ����һ�������������������ԭ�ӹ����������ӵ�P������Ԫ�ش���P>S>Si����2����̬Crԭ�ӵļ۵����Ų�ʽΪ3d54s1����λ�ڵ������ڵ�Moԭ�Ӽ۵����Ų�ʽΪ4d55s1����3��CH4��SiH4��ɡ��ṹ���ƣ�CH4����Է���������С�����Ӽ䷶�»���Ҳ��С������е�ϵͣ���4��[Ni(CN)4]2�����������Ӻ�����֮�������λ����������C��N֮��C��N���д���1���Ҽ���2���м������������Ӽ����������AE��ȷ����5��C60������̼ԭ��֮������γ��������κ�������Σ��������������С�CCC=120º��˵��̼ԭ�Ӳ�ȡsp2�ӻ�����6��������1����������Ϊ�о�����ÿ�����㱻����3���������ι��ã�ÿ���������κ��е�̼ԭ����=6��1/3=2��þԭ����Ϊ1��100%=1����ÿ���������λ���ϵĻ�ѧʽΪMgC2��

��ϰ��ϵ�д�
 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
 ����CH3(��)��C
����CH3(��)��C ������Ҫ���л���Ӧ�м���,�й����ǵ�˵������ȷ����(����)
������Ҫ���л���Ӧ�м���,�й����ǵ�˵������ȷ����(����)