��Ŀ����
����Ŀ�������������������������й㷺Ӧ�á�
��1����Ȼ���д��ڵ�54Fe��56Fe�����ǻ���Ϊ_____��
��2������Ƭ������Ũ�����У�Ƭ�̺���Ƭ��������ͭ��Һ�У�������Ƭ���������Ա仯��ԭ��_____��
��3����֪�������Ծ�ˮ��������ѧʽ_____���Խ�����ӷ�Ӧ����ʽ�����ֽ���������ˮ��ԭ��_____
��4����NaAlO2��Һ��AlCl3��Һ����ˮ��CO2����Ϊԭ�ϣ�ѡ������Լ���д��ʵ�����Ʊ�Al(OH)3���� �ӷ���ʽ������������;����_____��_____
��5����ϲ�dz��õ��к�θ���ҩ��仯ѧ�ɷ�������þ�ļ�ʽ�Σ���ѧʽΪAl2Mg6(OH)16CO3��4H2O���ɿ���2Al(OH)3��5Mg(OH)2��MgCO3��4H2O����1mol��ϲ�ֱ�������������� NaOH ��Һ��Ӧ����������� NaOH �����ʵ���֮��Ϊ_____��
���𰸡� ͬλ�� ��Ƭ����Ũ�����л�ۻ��������γ�����Ĥ��������ͭ������Ӧ����˷�������ͭ�������Ա仯 KAl(SO4)2��12H2O ��������ˮ��Al3+����ˮ�⣺Al3++3H2O![]() Al(OH)3+3H+�����ɵ�Al(OH)3���� ��ˮ�е������γɳ�����ʹˮ����� 2AlO2-+CO2+3H2O��2Al(OH)3��+CO32- Al3++3AlO2-+6H2O��4Al(OH)3����4Al3++3NH3��H2O��Al(OH)3��+NH4+ 9:2
Al(OH)3+3H+�����ɵ�Al(OH)3���� ��ˮ�е������γɳ�����ʹˮ����� 2AlO2-+CO2+3H2O��2Al(OH)3��+CO32- Al3++3AlO2-+6H2O��4Al(OH)3����4Al3++3NH3��H2O��Al(OH)3��+NH4+ 9:2
����������1��54Fe �� 56Fe����������ͬ����������ͬ�����ǻ�Ϊͬλ�ء�
��2���������Ũ���ᣬ�����γ���������Ĥ����ֹ����������ͭ�ķ�Ӧ������Ƭ���������Ա仯��
��3��������ѧʽΪKAl(SO4)2��12H2O����������ˮ������������ӣ������ӷ���ˮ�⣬Al3+ +3H2O![]() Al(OH)3(����)+3H+�����ɵ�Al(OH)3 ����������ˮ�е������γɳ������ﵽ����ˮ�ʵ�Ŀ�ġ�
Al(OH)3(����)+3H+�����ɵ�Al(OH)3 ����������ˮ�е������γɳ������ﵽ����ˮ�ʵ�Ŀ�ġ�
��4������һ��2AlO2-+CO2+3H2O==2Al(OH)3��+CO32- ����������4Al3++3NH3��H2O==Al(OH)3��+NH4+ ����������Al3++3 AlO2-+6H2O==4Al(OH)3��
��5��1mol ��ϲ[2Al(OH)3��5Mg(OH)2��MgCO3��4H2O]������������ʵ���Ϊ��6mol+10mol+2mol=18mol�������������Ƶ����ʵ���Ϊ��2mol+2mol=4mol����������� NaOH �����ʵ���֮��Ϊ9��2��

 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�����Ŀ������ʵ�鷽���ܴﵽʵ��Ŀ�ĵ����� ��
��� | A | B | C | D |
ʵ�鷽�� |
|
|
|
|
ʵ��Ŀ�� | ʵ�����Ʊ��������� | ���������ˮ | ��֤�����������������Ҵ���Һ�з�����ȥ��Ӧ��������ϩ | �ռ���ϩ����֤������ˮ�����ӳɷ�Ӧ |
A. A B. B C. C D. D
����Ŀ����֪���Ҷ��ᣨHOOC-COOH,�ɼ�дΪH2C2O4)�׳Ʋ���,1570Cʱ��ʼ�ֽ⣺
(1)̽�����������
250C H2C2O4 K1 = 5.4 x 10-2,K2 = 5. 4 x 10 -5 ��H2CO3 K1=4.5x10-7 K2= 4.7X10-11
���л�ѧ����ʽ������ȷ����________
A. H2C2O4 +CO32��=HCO3- +HC2O4�� B. HC2O4�� +CO32��= HCO3��+C2O42��
C. 2C2O42��+CO2+H2O=2HC2O4��+CO32�� D. H2C2O4 +CO32��=C2O42��+H2O+CO2
(2)̽������ֽ����
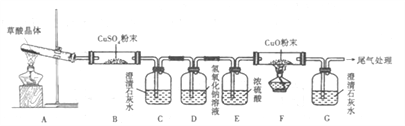
��ʵ���й۲쵽B��CuSO4��ĩ����,C�г���ʯ��ˮ�����,D������:_______��֤����CO�������ɵ������ǣ�_____________________
��д��H2C2O4�ֽ�Ļ�ѧ����ʽ_____________________
(3)̽�������Ի�ѧ��Ӧ���ʵ�Ӱ��
�ڼס�����֧�Թ��и�����4mLO.O1mol/T. KMnO4������Һ��2mL O.1mol/L H2C2O4��Һ�� �������Թ��м���һ���ƶ����MnSO4���壬ҡ�ȡ���д�±���
��Ӧ���� | ______________ |
ʵ����� | ______________ |
�Թ��з��ͷ�Ӧ�����ӷ���ʽ | ______________ |
(4)������KMnO4��Һ�ζ�Na2C2O4����Na2C2O4�Ĵ���
ʵ�鲽��:ȷ��ȡ2.OgNa2C2O4����,���1OO mL��Һ,ȡ��20.OOmL����ƿ��=����ƿ �м�������ϡH2SO4 ,��0.0160mol/L���Ը��������Һ�ζ����ζ����յ�ʱ���ĸ��������Һ25.OOmL0
�ٸ��������ҺӦװ��_______�ζ����С��������� ʽ�������� ʽ����
�ڵζ����յ�ʱ��ʵ�������ǣ�______________��
��Na2C2O4�Ĵ�����:______________