��Ŀ����
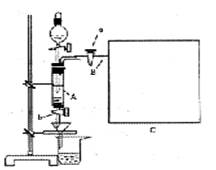
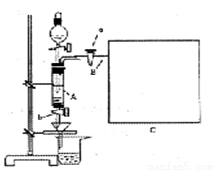
���Ȼ�������ѧ��ѧʵ�����г��õĻ�ѧ�Լ���ͬѧ�����÷���м��������ͭ���ʣ���̽���Ʊ�FeCl3��6H2O�ķ�����ͬѧ����Ƶ�ʵ��װ����ͼ��ʾ����ʵ�鲽�����£�A�з��з���м���ձ���ʢ�й�����ϡ���ᣬʵ��ʱ��a���ر�b���÷�Һ©����A�мӹ��������ᣬ��ʱ��Һ��dz��ɫ���ٴ�b���й��ˣ����˽�����ȡ�ձ�����Һ������������ȣ�����������ˮ��ʹʣ��HNO3�ֽ⣬�ٽ��½ᾧ��FeCl3��6H2O���塣��д���пհף�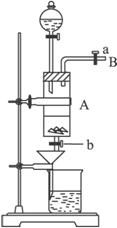
��1���μ�����ʱ�����ַ�Ӧ���ʽ�֮�����봿���۷�ӦҪ�죬��ԭ����__________________________________________________________________��
��2���ձ��ڷŹ���ϡHNO3��ԭ����_______________________________________________��
������Ӧ�����ӷ���ʽ��________________________________________________��
��3������ʵ������У����ɼ�a���������Ϊ�ų������������⣬��һ��Ŀ����________
____________________________________________________________________��
��4���������װ�ý���ʵ�飬����Ϊ��ʵ����ڵ�ȱ������У�
__________________________��__________________________����д�������ɣ�
��5����ͬѧ�Ը�ʵ������˸Ľ��������ÿ��ձ��н���Һ�������ʵ����Լ���Ȼ����HCl�������С�һ���¶���������Ũ�������½ᾧ���õ�������FeCl3��6H2O������Ϊ������Լ�������_____________��������ţ����������� ���������������ϡ���� �۸������������Һ ������������Һ
��HCl�������С�һ���¶���������Ũ�������½ᾧ��������__________________________
_____________________________________________________________________��
��1������м�к�����Cu���ʣ����������γ�ԭ��أ�ʹ��Ӧ���ʼӿ�
��2��ϡHNO3��ǿ�����ԣ�Ϊ��֤ʹFe2+ȫ��������Fe3+
3Fe2++4H++![]() ====3Fe3++NO��+2H2O
====3Fe3++NO��+2H2O
��3���������ͨ��ʹ��Һ©���е����Լ�A�е�Һ����˳������
��4����Ӧ�в������к��������Ⱦ���� ��ϡHNO3����FeCl2ʱ����Fe(NO3)3���ɶ�ʹ�Ƶõ�FeCl3��6H2O����������������Ҳ�ɣ�
��5���٢� ��ΪFeCl3+3H2O![]() Fe(OH)3+3HCl����������ʱHCl�ӷ����ˮ��ƽ��������Ӧ�����ƶ�������Fe(OH)3����HCl������������������ˮ����õ�FeCl3��6H2O
Fe(OH)3+3HCl����������ʱHCl�ӷ����ˮ��ƽ��������Ӧ�����ƶ�������Fe(OH)3����HCl������������������ˮ����õ�FeCl3��6H2O
������
��5��������һ�DZ�������������������������Ļ�ԭ�����H��Cl��O��Fe�ⲻ����������Ԫ�أ������Ǣ٢ڣ�

 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�