��Ŀ����
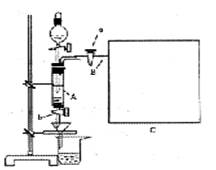
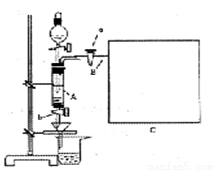
���Ȼ�������ѧ��ѧʵ�����бز����ٵ���Ҫ�Լ���ij��ȤС�����÷���м(������ͭ�Ȳ������ᷴӦ������)���Ʊ� FeCl3��6H2O����ͬѧ��Ƶ�ʵ��װ������ͼ��ʾ��A�з���m g����м���ձ���ʢ�й�����ϡ���ᣬʵ��ʱ��a���ر�b���ӷ�Һ©������A�мӹ�����ϡ���ᣬ��ʱ��Һ��dz��ɫ���ٴ�b ���й��ˣ����˽�����ȡ�ձ�����Һ������������ȣ�����������ˮ��ʹ����HNO3�ֽ⣬�ٽ��½ᾧ��FeCl3��6H2O���塣
��д���пհף�
(1)��μ��װ��A�������ԣ�________________________��
(2)�μ�����ʱ�����ַ�Ӧ���ʽ�֮ͬŨ�������봿���۷�ӦҪ�죬��ԭ����_____________
___________________��
(3)���ձ�����Һ��������Ũ�����ٽ��½ᾧ���Ƶ�FeCl3��6H2O���壬������ֱ�������ᾧ�ķ������Ƶþ����������_______________________________________________________��
(4)�ø÷��Ƶõľ�������������Fe (NO3)3��Ϊ���Ƶýϴ�����FeCl3��6H2O���ɽ��ձ��ڵ�ϡ���ỻ��________________________________________��
(5)��Ҫ�����м�Ĵ��ȣ��ɲ����B�зų���������V (������ɱ�״������λ��L)�������м�Ĵ���Ϊ__________(�ú�m��V�Ĵ���ʽ��ʾ)�����ڿ�ͼC�л�����Ҫ��װ�á�
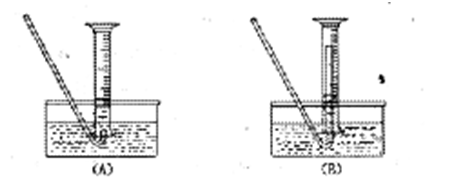
(1)�ٹرջ���a������b����Һ©��������ƿ�ǣ�����עˮ��©���¶˻��γ�һ��Һ����һ��ʱ�����Һ���������ƣ�˵�������Բ��ã���Һ��ֹͣ��������˵�������Ժá��ڹرշ�Һ©�������ͻ���b������a�����ܿ�B��һ�δ������ܵ��齺�ܲ�����ˮ���У���˫����סװ��A����ˮ���������ݲ������ſ�˫�ֲ��������γ�һ��Һ���������������á�
(2)����������ͭ�γ�ԭ��ط�Ӧ���ӿ��˷�Ӧ����
(3)��Ϊ�Ȼ�����ǿ��������ˮ�������������������ᣬ��������ʱʹHCl�ӷ����ˮ��ƽ�����ƣ���˵ò���FeCl3��6H2O����
(4)˫��ˮ��������ˮ
(5)![]() ��
��![]()

������(1)����װ�õ������Կ����ü��ȷ���עˮ����
(3)ֱ�������ᾧFeCl3��6H2Oʱ����ΪFeCl3+3H2O![]() Fe(OH)3+3HCl��HCl�ݳ�ʹ��ˮ��������У���˵ò���FeCl3��6H2O��
Fe(OH)3+3HCl��HCl�ݳ�ʹ��ˮ��������У���˵ò���FeCl3��6H2O��
(4)���������ʲ��ܽ�FeCl2������������Cl2��H2O2��
(5)����������������
Fe+2HCl![]() FeCl2+H2��
FeCl2+H2��
m(Fe) V L
![]()
����m(Fe)=![]()
�ʷ���м�Ĵ���Ϊ ��100%=
��100%=![]() ��100%
��100%