��Ŀ����
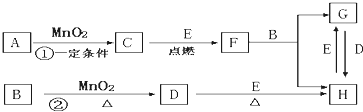
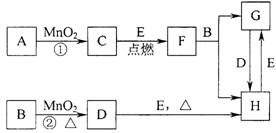
��ͼ���漰�����ʾ�Ϊ��ѧ��ѧ�еij������ʣ����У���C��D��E��F��Ϊ���ʣ�����Ϊ�������C��D��E �ڳ�����Ϊ���壬��������Ϊ�����Һ�壻��A��I ���ֻ��������ɫ��Ӧ�ֱ�Ϊ��ɫ�ͻ�ɫ���ܷ�Ӧ�١����е�һЩ�������Ѿ���ȥ����Щ��Ӧ������δ�г������Ǵ�������ת����ϵ��
�� д���й����ʵĻ�ѧʽ��
A ��D ��F ��I ��
�� ָ��MnO2����ط�Ӧ�е����ã���Ӧ������ ������Ӧ������ ����
�� д��B��MnO2���Ȼ��D�Ļ�ѧ����ʽ____________________________________��
��A��KClO3 D��Cl2 F��Na I��NaOH
�ƴ� ���� ��MnO2��4HCl(Ũ) =="=" MnCl2��Cl2����2H2O
����

��ϰ��ϵ�д�
�����Ŀ