��Ŀ����
��ͼʵ��װ��������֤ijЩ���ʵ����ʡ����Թ�A��װ�������Ĺ���NaHCO3,DΪ�̶������ӲֽƬ���Իش��������⣺
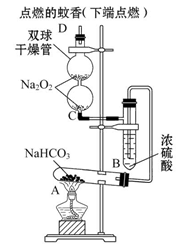
��1����ʵ���ʵ��Ŀ����__________________________________________��
��2����A�Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ��_______________________________��
��3��Bװ�õ�������_________________________________________________��
��4����˫�������ڷ�����Ӧ�Ļ�ѧ����ʽΪ____________________________��
��5��ʵ��ʱ�۲쵽��ʵ��������_______________________________________��
����ʵ������˵��___________________________________________________��
��6������������ڵ�Na2O2����Na2O����ʵ��ʱ�۲쵽��ʵ��������___________��

��1����ʵ���ʵ��Ŀ����__________________________________________��
��2����A�Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ��_______________________________��
��3��Bװ�õ�������_________________________________________________��
��4����˫�������ڷ�����Ӧ�Ļ�ѧ����ʽΪ____________________________��
��5��ʵ��ʱ�۲쵽��ʵ��������_______________________________________��
����ʵ������˵��___________________________________________________��
��6������������ڵ�Na2O2����Na2O����ʵ��ʱ�۲쵽��ʵ��������___________��
��1����֤CO2��Na2O2�ķ�Ӧ
��2��2NaHCO3 Na2CO3+H2O��+CO2��
Na2CO3+H2O��+CO2��
��3�����������е�ˮ����������CO2��
��4��2Na2O2+2CO2=2Na2CO3+O2
��5������ɫ��Na2O2��ת��Ϊ��ɫ��ĩ����ȼ������ȼ�ո��Ӿ��� Na2O2����CO2��Ӧ�����ɰ�ɫ��ĩ״���ʺ�O2
��6����ȼ��������Ϩ��
��2��2NaHCO3
 Na2CO3+H2O��+CO2��
Na2CO3+H2O��+CO2����3�����������е�ˮ����������CO2��
��4��2Na2O2+2CO2=2Na2CO3+O2
��5������ɫ��Na2O2��ת��Ϊ��ɫ��ĩ����ȼ������ȼ�ո��Ӿ��� Na2O2����CO2��Ӧ�����ɰ�ɫ��ĩ״���ʺ�O2
��6����ȼ��������Ϩ��
����ʵ��װ��ͼ����֪��ʵ������֤CO2��Na2O2�ķ�Ӧ����������ʵ��IJ����˳��Ϊ��A�е�NaHCO3���ȷֽ������CO2��H2O��g�������Թ�B��H2O��g����Ũ�������գ���B�г�����CO2������C��Na2O2��Ӧ������O2���Ӷ��ٽ������ȼ�ա��ڣ�6�����У���Na2O2����Na2O��������Ӧ��Na2O+CO2=Na2CO3����O2�ų��������������Ϩ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ