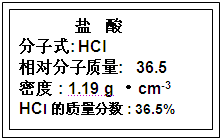
��Ŀ����
ȡ����Fe2O3��ĩ������ɫ�������������ᣬ�������ķ�Ӧ�Ļ�ѧ����ʽΪ��
��1��ȡ������Һ��һ֧�Թ��У�����NaOH��Һ���ɹ۲쵽�к��ɫ�������ɣ���Ӧ�����ӷ���ʽΪ
��2����С�ձ��м���25mL����ˮ�����������ں����ˮ�е��뼸��FeCl3������Һ�������������Һ��ɺ��ɫ�������Ƶ�
��3����ȡһ��С�ձ���Ҳ����25mL����ˮ�����ձ��м���1mLFeCl3��Һ����ҡ�Ⱥ����ձ����루2���е��ձ���һ������ڰ������ü�������䱭�е�Һ�壬�ɿ���
Fe2O3 +6HCl=2FeCl3 +3H2O
Fe2O3 +6HCl=2FeCl3 +3H2O
�÷�Ӧ�����ӷ���ʽΪFe2O3 +6H+=2Fe3++3H2O
Fe2O3 +6H+=2Fe3++3H2O
����Ӧ��������ҺΪ��
��
ɫ���ô���Һ�������·�Ӧ����1��ȡ������Һ��һ֧�Թ��У�����NaOH��Һ���ɹ۲쵽�к��ɫ�������ɣ���Ӧ�����ӷ���ʽΪ
Fe3++3 OH-=Fe��OH��3��
Fe3++3 OH-=Fe��OH��3��
����2����С�ձ��м���25mL����ˮ�����������ں����ˮ�е��뼸��FeCl3������Һ�������������Һ��ɺ��ɫ�������Ƶ�
������������
������������
����3����ȡһ��С�ձ���Ҳ����25mL����ˮ�����ձ��м���1mLFeCl3��Һ����ҡ�Ⱥ����ձ����루2���е��ձ���һ������ڰ������ü�������䱭�е�Һ�壬�ɿ���
��
��
�ձ��е�Һ���ж����ЧӦ�����������ݽ������������ᷴӦ�����κ�ˮ��д����Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��1�����������ж������д����Ӧ�����ӷ���ʽ��
��2��������ȡ�����ʵ�������
��3���������Һ�Dz�ͬ�ķ�ɢϵ�������ж����ЧӦ��Һû�У�
��1�����������ж������д����Ӧ�����ӷ���ʽ��
��2��������ȡ�����ʵ�������
��3���������Һ�Dz�ͬ�ķ�ɢϵ�������ж����ЧӦ��Һû�У�
����⣺Fe2O3��ĩ������ɫ�������������ᣬ�������ķ�Ӧ�Ļ�ѧ����ʽΪ��Fe2O3 +6HCl=2FeCl3 +3H2O���÷�Ӧ�����ӷ���ʽΪ��Fe2O3 +6H+=2Fe3++3H2O��������Һʵ������ҺFeCl3����Һ�к���Fe3+���ӣ���Һ�ʻ�ɫ��
�ʴ�Ϊ��Fe2O3 +6HCl=2FeCl3 +3H2O
Fe2O3 +6H+=2Fe3++3H2O�� �ƣ�
��1��ȡ��������Һ������NaOH��Һ��������Ӧ��Fe3++3OH-=Fe��OH��3�����ɹ۲쵽�к��ɫ����Fe��OH��3���ɣ�
�ʴ�Ϊ��Fe3++3 OH-=Fe��OH��3����
��2�����ˮ�е��뼸��FeCl3������Һ�������������Һ��ɺ��ɫ�������Ƶ������������壬
�ʴ�Ϊ�������������壻
��3��������ж����ЧӦ���ʴ�Ϊ���ң�
�ʴ�Ϊ��Fe2O3 +6HCl=2FeCl3 +3H2O
Fe2O3 +6H+=2Fe3++3H2O�� �ƣ�
��1��ȡ��������Һ������NaOH��Һ��������Ӧ��Fe3++3OH-=Fe��OH��3�����ɹ۲쵽�к��ɫ����Fe��OH��3���ɣ�
�ʴ�Ϊ��Fe3++3 OH-=Fe��OH��3����
��2�����ˮ�е��뼸��FeCl3������Һ�������������Һ��ɺ��ɫ�������Ƶ������������壬
�ʴ�Ϊ�������������壻
��3��������ж����ЧӦ���ʴ�Ϊ���ң�
���������ӷ���ʽ����дҪע�⣺
1��ԭ���غ㡢����غ㣻
2��������ʡ����ʡ����塢������������д��ѧʽ��
3��������Ϊ��Ӧ�Ũ�ȴ�ʱд��ѧʽ����ϡ��Һд����ʽ����������ʱ��һ�㰴�����ﴦ����д��ѧʽ��
1��ԭ���غ㡢����غ㣻
2��������ʡ����ʡ����塢������������д��ѧʽ��
3��������Ϊ��Ӧ�Ũ�ȴ�ʱд��ѧʽ����ϡ��Һд����ʽ����������ʱ��һ�㰴�����ﴦ����д��ѧʽ��

��ϰ��ϵ�д�
�����Ŀ