��Ŀ����
ȡ����Fe2O3��ĩ������ɫ�������������ᣬ�õ���ɫ��Һ���ô���Һ��������ʵ�飺
��1��д��������ȡ��ɫ��Һ�Ļ�ѧ����ʽ��
��2����С�ձ��м���20mL����ˮ�����������ں����ˮ�м�����ȡ�Ļ�ɫ��Һ2mL�����������Һ���
��3���ü���������ձ��е�Һ�壬���Թ۲쵽Һ����
��4�����ձ�����μ�����������ᣬ�����һϵ�б仯��
���ȳ���
�����
��1��д��������ȡ��ɫ��Һ�Ļ�ѧ����ʽ��
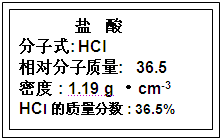
Fe2O3 +6HCl=2FeCl3 +3H2O
Fe2O3 +6HCl=2FeCl3 +3H2O
����2����С�ձ��м���20mL����ˮ�����������ں����ˮ�м�����ȡ�Ļ�ɫ��Һ2mL�����������Һ���
���
���
ɫ��ֹͣ���ȣ��Ƶõķ�ɢϵΪ������������
������������
���÷�ɢϵ����ֱ���ķ�Χ��1nm��100
1nm��100
nm����3���ü���������ձ��е�Һ�壬���Թ۲쵽Һ����
�����ЧӦ
�����ЧӦ
�������ʵ�������������
����
����Һ
��Һ
����4�����ձ�����μ�����������ᣬ�����һϵ�б仯��
���ȳ���
���ɫ����
���ɫ����
��ԭ���ǣ�����ʹ������������۳���
����ʹ������������۳���
�������
�����ܽ�
�����ܽ�
��ԭ���ǣ�Fe��OH��3+3H+=Fe3++3H2O
Fe��OH��3+3H+=Fe3++3H2O
���������ӷ���ʽ��ʾ����������1�����ݽ��������������ᷴӦ�����κ�ˮ��
��2��������ȡ�����ʵ�������
��3����������������ж����ЧӦ��Һû�ж����ЧӦ��
��4���������������彺���������ᣨ�������Һ���ᷢ���۳���
���������������������������ᣬ�ᷢ������кͷ�Ӧ��
��2��������ȡ�����ʵ�������
��3����������������ж����ЧӦ��Һû�ж����ЧӦ��
��4���������������彺���������ᣨ�������Һ���ᷢ���۳���
���������������������������ᣬ�ᷢ������кͷ�Ӧ��
����⣺��1�����������������ᷴӦ�����κ�ˮ��Fe2O3��ĩ�����������ᣬ�����ķ�Ӧ��Fe2O3 +6HCl=2FeCl3 +3H2O��
�ʴ�Ϊ��Fe2O3 +6HCl=2FeCl3 +3H2O��
��2�����ˮ�е��뼸��FeCl3������Һ�������������Һ��ɺ��ɫ�������Ƶ������������壬��ӦΪFe3++3H2O?Fe��OH��3�����壩+3H+�������з�ɢ������ֱ����1nm��100nm֮�䣬
�ʴ�Ϊ����֣������������壻1nm��100��
��3���ü���������ձ��е�Һ�壬�������Ĵ�ֱ������Թ۲쵽Һ���г��ֵ�һ�������ġ�ͨ·��������Ƕ����ЧӦ����������������ж����ЧӦ����Һû�ж����ЧӦ�������ö����ЧӦ�����ֽ������Һ��
�ʴ�Ϊ�������ЧӦ�����塢��Һ��
��4������ʹ����۳��ķ����У�������෴��ɽ����Ľ��塢���ȡ�����������Һ�������������彺���������ᣨ�������Һ���ᷢ���۳����������ɫ��������������
�ʴ�Ϊ�����ɫ����������ʹ������������۳���
��������������۳��������������������������������ᣬ�ᷢ������кͷ�Ӧ�������Ȼ�����ˮ�����ӷ���ʽΪFe��OH��3+3H+=Fe3++3H2O��
�ʴ�Ϊ�������ܽ⣻Fe��OH��3+3H+=Fe3++3H2O��
�ʴ�Ϊ��Fe2O3 +6HCl=2FeCl3 +3H2O��
��2�����ˮ�е��뼸��FeCl3������Һ�������������Һ��ɺ��ɫ�������Ƶ������������壬��ӦΪFe3++3H2O?Fe��OH��3�����壩+3H+�������з�ɢ������ֱ����1nm��100nm֮�䣬
�ʴ�Ϊ����֣������������壻1nm��100��
��3���ü���������ձ��е�Һ�壬�������Ĵ�ֱ������Թ۲쵽Һ���г��ֵ�һ�������ġ�ͨ·��������Ƕ����ЧӦ����������������ж����ЧӦ����Һû�ж����ЧӦ�������ö����ЧӦ�����ֽ������Һ��
�ʴ�Ϊ�������ЧӦ�����塢��Һ��
��4������ʹ����۳��ķ����У�������෴��ɽ����Ľ��塢���ȡ�����������Һ�������������彺���������ᣨ�������Һ���ᷢ���۳����������ɫ��������������
�ʴ�Ϊ�����ɫ����������ʹ������������۳���
��������������۳��������������������������������ᣬ�ᷢ������кͷ�Ӧ�������Ȼ�����ˮ�����ӷ���ʽΪFe��OH��3+3H+=Fe3++3H2O��
�ʴ�Ϊ�������ܽ⣻Fe��OH��3+3H+=Fe3++3H2O��
���������⿼�齺���н������ӵ�ֱ������������ʣ�ע�ⶡ���ЧӦ�ǽ������е����ʡ����ս�����Ʊ��ǽ����Ĺؼ�����Ŀ�ϼ�

��ϰ��ϵ�д�
�����Ŀ