��Ŀ����
ijѧ������֪���ʵ���Ũ�ȵ�����ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱѡ�������ָʾ��������д���пհף�
��1�����ƴ���Һ���ú����������ʣ����ʲ������ᷴӦ���Ĺ����ռ���Ʒ����500mL��Һ�����ձ�����Ͳ����ͷ�ιܺͲ������⣬����Ҫ�IJ��������� ��
��2�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���________��
| A����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��� |
| B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и��� |
| C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ |
| D����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������� |
| �ζ����� | ����NaOH ��Һ������� | 0.1000mol/L��������/mL[��Դ:ѧ+ | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.28 | 26.28 |
| �ڶ��� | 25.00 | 1.55 | 30.30 | 28.75 |
| ������ | 25.00 | 0.20 | 26.42 | 26.22 |
��4���ñ�������ζ����������NaOH��Һʱ���۾�Ҫע����ƿ����Һ��ɫ�ı仯��ֱ�������һ���������Һ�ɻ�ɫ��________ɫ������___________________Ϊֹ��
��ÿ��2�֣���10�֣�
��1��500mL����ƿ������ƿ��ע����÷�) (2��)��
��2�� A (2��)
��3�� V="26.25mL" , c(NaOH)=0.1000mol��L-1��26.25mL/25.00mL="0.1050mol/L" (��Ϊ0.105 mol/L ��1�֣������𰸲��÷�) (2��)
��4�� �� (2��)������Ӳ���ɫ (2��)
���������������1��������Һ����IJ��������У��ձ�����Ͳ����ͷ�ιܡ�������������ƿ��
��2��A���ʼ���Ӷ������������ƫС���ζ�����ʱ���Ӷ������������ƫ��������������ƫС����ʹ����NaOH��Һ��Ũ����ֵƫ�ͣ�����ȷ��
��3���ڶ������������̫����ȥ���õ�һ�κ͵����ε��������м��㣬 V="26.25mL" , c(NaOH)=0.1000mol��L-1��26.25mL/25.00mL=0.1050mol/L��
��4���ζ��������жϡ�����ζ�����������Һ�ɻ�ɫ���ɫ�����Ұ���Ӳ���ɫΪֹ��
���㣺����һ�����ʵ���Ũ�ȵ���Һ ����к͵ζ�
���������⿼��������к͵ζ����ѶȲ���ע�����ζ��ܵ�С�̶����ϣ���̶����£���ȷ����ʱ��Һ�����ƫ����ƫС��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�ijѧ������֪���ʵ���Ũ�ȵ�����ⶨδ֪���ʵ�/��Ũ�ȵ�NaOH��Һʱѡ�������ָʾ��������д���пհף�
��1�ñ�������ζ������NaOH��Һʱ������_______________________,����_____________________�۾�ע��__________________________________ֱ�������һ���������Һ�ɻ�ɫ��______ɫ������_____________________Ϊֹ��
��2�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵��ǣ���
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ������ ���ζ���������ʧ
���ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ������ʼ����Ϊ_____mL�յ����Ϊ_______mL,����������Һ�����Ϊ_________mL
��4��ijѧ������3��ʵ��ֱ��¼�й��������±���
| �ζ����� | ����NaOH��Һ����� | 0.1000mol/L��������/mL[��Դ:ѧ+��+��] | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һ��
������ɷֽ�Ϊ���¼�����
a. ��ȡ20.00mL�����NaOH��Һע��ྻ����ƿ��������2-3�η�̪
b. �ñ�������Һ��ϴ�ζ���2-3��
c. ��ʢ�б���Һ����ʽ�ζ��̶ܹ��ã�����Һ��ʹ�ζ��ܼ��������Һ
d. ȡ��������Һע����ʽ�ζ�����0�̶�����2-3cm
e. ����Һ����0��0�̶����£����¶���
f. ����ƿ���ڵζ��ܵ����棬�ñ�������Һ�ζ����յ㣬���µζ���Һ��Ŀ̶�
���������գ�
��1����ȷ������˳���ǣ��������ĸ��д��__________________ ____��
��2���ζ��յ�ʱ��Һ����ɫ�仯�� ��
��3�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���_____ ___��
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��4����ij�εζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����Ӧ������������Ϊ________ mL��
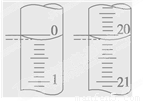
��5��ijѧ������3��ʵ��ֱ��¼�й��������±���
|
�ζ� ���� |
����NaOH��Һ�����/mL |
0.1000mol/L��������/mL |
||
|
�ζ�ǰ�̶� |
�ζ���̶� |
��Һ���/mL |
||
|
��һ�� |
25.00 |
0.20 |
20.22 |
|
|
�ڶ��� |
25.00 |
0.56 |
24.54 |
|
|
������ |
25.00 |
0.42 |
20.40 |
|
�����ϱ��������NaOH��Һ�����ʵ���Ũ��Ϊ ��
ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�������ָʾ��������д���пհף�
��1���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע�� ��ֱ��������һ���������Һ�� ɫ��Ϊ ���� Ϊֹ��
��2�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���________
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����Ӧ������������Ϊ________ mL��
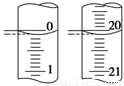
��4��ijѧ������3��ʵ��ֱ��¼�й��������±���
|
�ζ� ���� |
����NaOH��Һ�����/mL |
0��100 mol/L��������/mL |
||
|
�ζ�ǰ�̶� |
�ζ���̶� |
��Һ���/mL |
||
|
��һ�� |
25��00 |
0��20 |
20��22 |
|
|
�ڶ��� |
25��00 |
0��56 |
24��54 |
|
|
������ |
25��00 |
0��42 |
20��40 |
|
�����ϱ��������NaOH��Һ�����ʵ���Ũ��Ϊ ��
ijѧ������֪���ʵ���Ũ�ȵ�����ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱѡ�������ָʾ��������д���пհף�
��1�����ƴ���Һ���ú����������ʣ����ʲ������ᷴӦ���Ĺ����ռ���Ʒ����500mL��Һ�����ձ�����Ͳ����ͷ�ιܺͲ������⣬����Ҫ�IJ��������� ��
��2�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���________��
A����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
��3��ijѧ������3��ʵ��ֱ��¼�й��������±���
|
����� |
����NaOH ��Һ������� |
0.1000mol/L��������/mL[��Դ:ѧ+ |
||
|
�ζ�ǰ�̶� |
�ζ���̶� |
��Һ���/mL |
||
|
��һ�� |
25.00 |
0.00 |
26.28 |
26.28 |
|
�ڶ��� |
25.00 |
1.55 |
30.30 |
28.75 |
|
������ |
25.00 |
0.20 |
26.42 |
26.22 |
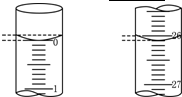
�����ϱ�����Ч���ݼ����NaOH��Һ�����ʵ���Ũ�ȣ�c(NaOH)=__________��(������λ��Ч����)
��4���ñ�������ζ����������NaOH��Һʱ���۾�Ҫע����ƿ����Һ��ɫ�ı仯��ֱ�������һ���������Һ�ɻ�ɫ��________ɫ������___________________Ϊֹ��
 ijѧ������֪���ʵ���Ũ�ȵ��������ζ�δ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�
ijѧ������֪���ʵ���Ũ�ȵ��������ζ�δ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�