��Ŀ����
������أ�K2FeO4����һ�����͡���Ч�������ɫˮ����������C12��O2��C1O2��KMnO4�����Ը�ǿ��������Ⱦ����ҵ�������Ƶø������ƣ�Ȼ���ڵ����£������������Һ�м���KOH�����ͣ�ʹ�������������
��1���ɷ��Ʊ�������ص���Ҫ��ӦΪ��
2FeSO4 + 6Na2O2= 2Na2FeO4 + 2Na2O + 2Na2SO4 + O2��
�ٸ÷�Ӧ�е��������� ����ԭ���� ��ÿ����l molNa2FeO4ת�� mol���ӡ�
�ڼ�Ҫ˵��K2FeO4��Ϊˮ������ʱ��������� ��
��
��2��ʪ���Ʊ�������أ�K2FeO4���ķ�Ӧ��ϵ��������������Fe(OH)3��C1O����OH����FeO42-��C1����H2O��
��д������ƽʪ���Ƹ�����ص����ӷ�Ӧ����ʽ�� ��
��ÿ����1mol FeO42- ת�� mo1���ӣ�����Ӧ������ת����0.3mo1���ӣ���ԭ��������ʵ���Ϊ mo1��
�۵����£��ڸ���������Һ�м���KOH�����Ϳ�����������أ�K2FeO4����˵��ʲô���� ��
��1����Na2O2�� Na2O2��FeSO4��5
�ڸ�����ؾ���ǿ�����ԣ���ɱ��������������������������ԭΪFe3+��Fe3+ˮ������Fe(OH)3����������ˮ���������ʶ�������
��2����2Fe(OH)3+3ClO?+4OH?=2FeO42?+3Cl?+5H2O
��3;0.15mol
�۸��¶���K2FeO4��Na2FeO4���ܽ��С��
���������������1����6Na2O2��,10��O��-1�۽���Ϊ-2�ۣ�����Na2O2Ϊ��������FeSO4�е�Fe��+2������Ϊ+6�ۣ�6Na2O2��,2��O��-1������Ϊ0����Na2O2��FeSO4Ϊ��ԭ��������l molNa2FeO4ʱ��5molO��-1�۽���Ϊ-2�ۣ�ת�Ƶ���5mol����ȫ�濼�����⣬��Ҫ���ǵ�K2FeO4����ǿ�����ԣ���Ҫ���Dz���Fe3+�����ʡ�
��2����C1O-��Fe(OH)3����ΪFeO42?��Cl����ΪCl?������Ԫ����ɣ���Ӧ����OH?����������H2O����ƽ�ɵ����ӷ���ʽ����Fe��+6�۽�Ϊ+3�ۣ�����ÿ����1mol FeO42- ת��3mol���ӣ����ݻ��ϼ۵ı仯�ͷ���ʽ��֪��Cl?��2e?��ת��0.3mo1����ʱ����ԭ����Cl?Ϊ0.15mol��
�۸��ݳ����ܽ�ƽ�⣬����Һ�У��ܽ��С��������������
���㣺���⿼����������ԭ�����жϡ�����ת�Ƽ����㡢���ӷ���ʽ����д�������ܽ�ƽ�⡣

 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д���1���ü���������һ�����۵����ⷽ�����йص��Ȼ�ѧ����ʽ����:
CH4(g)+1/2O2(g)  CO(g)+2H2(g)����H= ��36kJ/mol������ ��
CO(g)+2H2(g)����H= ��36kJ/mol������ ��
CH4(g)+H2O(g)  CO(g)+3H2(g)����H= +216kJ/mol����������
CO(g)+3H2(g)����H= +216kJ/mol����������
������˵����ȷ���� ____
| A��2H2O(l)=2H2(g)+O2(g) ��H=+504kJ/mol |
| B������Ӧ���Ц�(CH4)��=��(CO)��ʱ�������÷�Ӧ��ƽ��״̬ |
| C��������������ʱ��������ϵѹǿ����Ӧ�٢��м����ת���ʾ����� |
| D��Ϊά�ֺ㶨�¶ȣ�������������ʧ����ij��Ӧ����ͬʱ������Ӧ�����ʱ�����ļ� |
��ij�¶��£���100L��Ӧ���г�������ˮ���������ʵ����ֱ�Ϊ100mol��300molʱ���ٶ�ֻ������ӦCH4(g)+H2O(g)
 CO(g)+3H2(g)���������ת����Ϊ0.5ʱ����ʱƽ�ⳣ��Ϊ _______
CO(g)+3H2(g)���������ת����Ϊ0.5ʱ����ʱƽ�ⳣ��Ϊ _______��2������Һ����NaNO3��NaNO2��NaOH��ɵģ��ڼ��������£�ͨ��������Ӧʹ����������һ�����ܵ�Fe3O4��
3Fe+NaNO2+5NaOH
 3Na2FeO2+H2O+NH3�����������٣�
3Na2FeO2+H2O+NH3�����������٣�8Fe+3NaNO3+5NaOH+2H2O
 4Na2Fe2O4+3NH3�������ڣ�
4Na2Fe2O4+3NH3�������ڣ��Լ������������������������벹���������Ӧ�ķ���ʽ����ƽ�� __________________
���û�ѧԭ���Թ����ŷŵķ�ˮ�������Ƚ�����Ч��������������
(һ)Ⱦ�Ϲ�ҵ�ŷŵķ�ˮ�к��д����ж���NO2-�������ڼ��������¼������۳�ȥ(���ȴ�����ķ�ˮ�������ʹʪ��ĺ�ɫʯ����ֽ����������)����ȥNO2-�����ӷ���ʽΪ________��
(��)ij�������Ƹ﹤ҵ������Cr(��)�����������ù�������(�����ȡҺ�н���������Ҫ��Cr3���������Fe3����Fe2����Al3����Ca2����Mg2��)��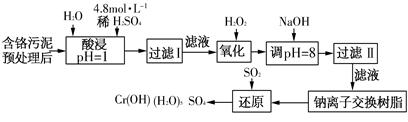
�����²���������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe3�� | Fe2�� | Mg2�� | Al3�� | Cu2�� | Cr3�� |
| ��ʼ���� ʱ��pH | 1.9 | 7.0 | �� | �� | 4.7 | �� |
| ������ȫ ʱ��pH | 3.2 | 9.0 | 11.1 | 8 | 6.7 | 9(>9�ܽ�) |
(1)���ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ��________(����дһ��)��
(2)��pH��8��Ϊ�˳�ȥ________(��Fe3����Al3����Ca2����Mg2��)��
(3)�����ӽ�����֬��ԭ��ΪMn����nNaR��MRn��nNa����������������������________(��Fe3����Al3����Ca2����Mg2��)��
(4)����ƽ������ԭ��Ӧ����ʽ����Na2Cr2O7����SO2����H2O = ��Cr(OH)(H2O)5SO4����Na2SO4��ÿ����1mol Cr(OH)(H2O)5SO4����SO2�����ʵ���Ϊ________��
(��)ӡˢ��·ͭ�帯ʴ������FeCl3����ʴͭ���Ļ����Һ�У���Cu2����Fe3����Fe2����Ũ�Ⱦ�Ϊ0.10mol��L��1��������ϱ����������ݺ��ṩ��ҩƷ��������ȥCuCl2��Һ��Fe3����Fe2����ʵ�鲽�裺��________����________���۹��ˡ�(�ṩ��ҩƷ��Cl2��ŨH2SO4��NaOH��Һ��CuO��Cu)��
KMnO4��Һ������������ԭ��Ӧ�ζ��ı�Һ������KMnO4��ǿ�����ԣ�������Һ�����ױ������л�ˮ�е�ijЩ������ԭ�����ʻ�ԭ����������������MnO(OH)2���������KMnO4����Һ�IJ���������ʾ���ٳ�ȡ�Զ�����������KMnO4��������ˮ�У�����Һ���Ȳ�������1 h�������ײ���©�����˳�ȥ���ܵ�MnO(OH)2���۹��˵õ���KMnO4��Һ��������ɫ�Լ�ƿ�����ڰ�����������������ԭ�ζ��������û��Լ�(���ȸߡ���Է��������ϴ��ȶ��ԽϺõ�����)��Һ�궨��Ũ�ȣ�KMnO4�ڵζ��б���ԭ��Mn2+����ش��������⣺
��1�� ȷ��ȡһ�������KMnO4��Һ��Ҫʹ�õ�������____________��
��2�� �����������У����ڱ궨KMnO4��Һ�Ļ��Լ����ѡ��________(�����)��
| A����������Ϊ30%��˫��ˮ | B��FeSO4 | C��Ħ���� | D��Na2SO3 |
��4�� ����H2C2O4��2H2O�����Լ���������ԭ�ζ�������ʱ��Ӧ���������Է�Ӧ��Ҫ��70��80 �������½��У����¶ȸ��ᵼ�²���ֽ�������壬д������ֽ�Ļ�ѧ��Ӧ����ʽ �����������õĸ�����ر�ҺŨ�Ȼ� (�ƫ�ߡ�����ƫ�͡�����Ӱ�족)����ʹ����70��80 ��������һ��ʼ�ζ�ʱ��Ӧ��Ȼ���������μ�KMnO4��Һ����Ļ��������KMnO4��ҺŨ�Ⱦֲ����߶��ֽ⣬����ڿ�ʼ���β���ʱӦ ���ڵ��뼸��KMnO4��Һ֮��Ӧ���ʻ�Ѹ�ټӿ죬�õζ�����Ҳ����Ӧ�ӿ죬��Ӧ���ʼӿ��ԭ���� ��
��5�����÷������ܺ��KMnO4����Һȥ�ⶨˮ����Fe2���ĺ�������õ�Fe2��Ũ��ֵ��________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��