��Ŀ����
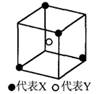
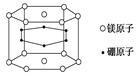
��1��+3��Co�������CoClm��nNH3������ԭ�ӵ���λ��Ϊ6����1 mol�������������AgNO3��Һ��Ӧ����1 mol AgCl���������������ʽд���������Ļ�ѧʽ ��
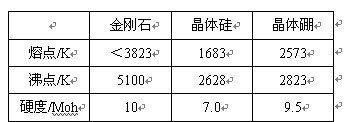
��2���о����ʴ��Ա��������������Ӻ�δ�ɶԵ���Խ�࣬�����Խ��ż�¼����Խ�á�������������MnO2��Fe3O4��Cr2O3�У�����������___________��
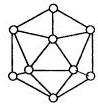
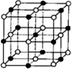
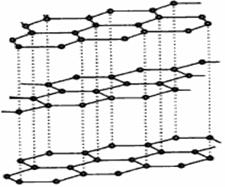
��3��̼�����е������ʯī��������ɣ���ṹ������ʯī��������ͼ����ÿ��̼ԭ��ͨ���� ���ӻ�����Χ̼ԭ�ӳɼ���
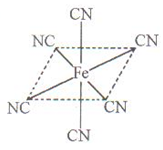
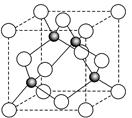
��4����Ԫ��Ӧ�ù㷺��Fe2+��KCN��Һ��Ӧ��Fe��CN��2���������������KCN��Һʱ�����ܽ⣬���ɻ�Ѫ�Σ��������ӽṹ������ͼ��
����֪CN����N2�ṹ���ƣ�1molCN����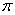 ����ĿΪ ��
����ĿΪ ��
�����������ܽ���̵Ļ�ѧ����ʽΪ ��
��2���о����ʴ��Ա��������������Ӻ�δ�ɶԵ���Խ�࣬�����Խ��ż�¼����Խ�á�������������MnO2��Fe3O4��Cr2O3�У�����������___________��
��3��̼�����е������ʯī��������ɣ���ṹ������ʯī��������ͼ����ÿ��̼ԭ��ͨ���� ���ӻ�����Χ̼ԭ�ӳɼ���


��4����Ԫ��Ӧ�ù㷺��Fe2+��KCN��Һ��Ӧ��Fe��CN��2���������������KCN��Һʱ�����ܽ⣬���ɻ�Ѫ�Σ��������ӽṹ������ͼ��
����֪CN����N2�ṹ���ƣ�1molCN����
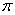 ����ĿΪ ��
����ĿΪ �������������ܽ���̵Ļ�ѧ����ʽΪ ��
��10�֣���1��[CoCl2(NH3)4]Cl��2��Fe3O4��3��sp2 ��4����2NA
�� ����2�֣�
����2�֣�
��
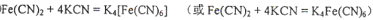 ����2�֣�
����2�֣������������1��1 mol�������������AgNO3��Һ��Ӧ����1 mol AgCl��������˵������λ����������1�������Ӳ������塣����ΪCr�ǣ�3�۵ģ�����ԭ�ӵĹ�����3����������λ����6�����Ի�ѧʽӦ����[CoCl2(NH3)4]Cl��
��2�����ݺ�������Ų�ʽ��֪��������������MnO2��Fe3O4��Cr2O3�н��������Ӻ��е�δ�ɶԵ��ӷֱ���3��14/3��3�����Դ�������Fe3O4��
��3��ʯī�Dz�״�ṹ������ÿ��̼ԭ��ͨ��sp2�ӻ�����Χ̼ԭ�ӳɼ���
��CN����N2�ṹ���ƣ������к�����������2��
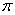 ��������1molCN����
��������1molCN����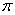 ����ĿΪ2NA��
����ĿΪ2NA���ڸ��ݽṹʽ��֪�������к�����λ��������λ����6�����Գ����ܽ���̵Ļ�ѧ����ʽΪFe(CN)2��4KCN��K4Fe(CN)6��
 �����жϺ��йؼ���
�����жϺ��йؼ��������������Ǹ߿��еij������ͺͿ��㣬�����е��Ѷ�����Ŀ��顣�����ۺ���ǿ����ע�ض�ѧ������֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ�����������ͷ�����������ѵ��������������ѧ���������������ͷ�ɢ˼ά���������ѧ��������û���֪ʶ���ʵ�������������ּ�ڿ���ѧ����֪ʶ�����������á�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ