��Ŀ����
13��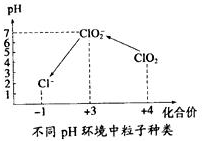 �������ȣ�C1O2��������һ�ֳ��ø�Ч������ˮ��������
�������ȣ�C1O2��������һ�ֳ��ø�Ч������ˮ����������1��KC1O3��Ũ������һ���¶��·�Ӧ������C1O2����Ӧ����Ϊ2C1O3ʮ4HC1��Ũ��=2KC1+2C1O2��+Cl2��+2H2O��Ũ�����ڸ÷�Ӧ�б��ֳ��������ǻ�ԭ�Ժ����ԣ�
��2��ʵ���ҳ���KC1O3�����ᣨH2C2O4����ϡ�����Ʊ�C1O2���÷�Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ1��1��
��3����C1O2ͨ�뵽������Һ�У�Ȼ�����������ϡ�����ữ���Ȼ�����Һ�������а�ɫ�������ɣ�д������������������Һ��Ӧ�����ӷ���ʽ5H2S+8ClO2+4H2O=18H++5SO42-+8Cl-��
��4��C1O2��Cl2������ʱ����������ԭΪClһ�����³�ѹ�£��������C1O2����������Cl2��2.5����
��5������ˮ����ClO2�������ˮ�У�Ҫ��ClO2��Ũ����0.1-0.8mg•L-1֮�䣮���������Լ��ˮ��ClO2��Ũ�ȣ���ͬpH������������������ͼ��ʾ�����������£�
I��ȡһ�������ˮ��������һ�����ĵ⻯�أ��ٽ���Ӧ����Һ�������ԣ������������Һ����Һ������
II������һ������Na2S2O3��Һ������֪��2S2O32-+I2=S4O62-+2I-��
III�����������ˮ��pH��1��3��
���ʴ�
�ٲ���I�з�Ӧ�����ӷ���ʽ��2ClO2+2I-=2ClO2-+I2��
���ڲ���III�����У���Һ�ֳ���ɫ����Ӧ�����ӷ���ʽ��ClO2-+4I-+4H+=Cl-+2I2+2H2O��
����ˮ�������Ϊ1.0L���ڲ���IIʱ������1.0��10-3mol•L-1����Na2S2O3��Һ10mL����ˮ����C1O2��Ũ����0.675mg•L-1��
���� ��1������KC1O3��Ũ���ᷴӦ�ķ���ʽ��֪��Ũ�����в�����Ԫ�صĻ��ϼ۴�-1��Ϊ0�ۣ����в��������Ȼ��أ��ݴ˴��⣻
��2��KClO3�Ͳ��ᣨH2C2O4�������������·�Ӧ����ClO2��CO2��KHSO4������������ԭ��Ӧ����������غ����д��ѧ����ʽ����Ϸ���ʽ�ж���������ͻ�ԭ��������ʵ���֮�ȣ�
��3�����ᱵ���������ᣬ����Ӧ�������ᱵ��C1O2ͨ�뵽������Һ�У�����������������ݵ��ӵ�ʧ�غ���д���ӷ�Ӧ����ʽ���ɣ�
��4��molC1O2��Cl2������ʱ����������ԭΪCl-��ÿmolC1O2����ԭΪCl-��Ҫ��5mol���ӣ�ÿmolC12����ԭΪCl-��Ҫ��2mol���ӣ����ݵ���ת����Ŀ��ȴ��⣻
��5����������������Һ�������ԣ���ͼ֪������������������Ϊ ClO2��ClO2������ĵ⻯������ΪI2����������ԭ��ClO2-�����ݻ��ϼ�����������ȡ�ԭ���غ�͵���غ�����ƽ��
����ͼʾ������pH��1��3ʱ��ClO2-��I-������I�����ɵ�I2����۽���ٴγ�����ɫ�����ݻ��ϼ�����������ȡ�ԭ���غ�͵���غ�����ƽ��
�۸��ݹ�ϵS2O32-��I-��ClO2�ȼ����ClO2�����ʵ�����Ȼ���ټ����Ũ��
��� �⣺��1������KC1O3��Ũ���ᷴӦ�ķ���ʽ��֪��Ũ�����в�����Ԫ�صĻ��ϼ۴�-1��Ϊ0�ۣ����в��������Ȼ��أ�����Ũ�����ڸ÷�Ӧ�б��ֳ��������ǻ�ԭ�Ժ����ԣ�
�ʴ�Ϊ����ԭ�Ժ����ԣ�
��2��KClO3�Ͳ��ᣨH2C2O4�������������·�Ӧ����ClO2��CO2��KHSO4����Ӧ�ķ���ʽΪ2KClO3+H2C2O4+2H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$2ClO2��+2CO2��+2KHSO4+2H2O����������ΪCO2����ԭ����ΪClO2���������ʵ���֮��Ϊ1��1��
�ʴ�Ϊ��1��1��
��3�����ᱵ���������ᣬ����Ӧ�������ᱵ��C1O2ͨ�뵽������Һ�У���������������������ӷ�Ӧ����ʽΪ��5H2S+8ClO2+4H2O=18H++5SO42-+8Cl-��
�ʴ�Ϊ��5H2S+8ClO2+4H2O=18H++5SO42-+8Cl-��
��4��molC1O2��Cl2������ʱ����������ԭΪCl-��ÿmolC1O2����ԭΪCl-��Ҫ��5mol���ӣ�ÿmolC12����ԭΪCl-��Ҫ��2mol���ӣ����ݵ���ת����Ŀ��ȿ�֪�������C1O2����������Cl2��2.5����
�ʴ�Ϊ��2.5��
��5����������������Һ�������ԣ���ͼ֪������������������Ϊ ClO2��ClO2������ĵ⻯������ΪI2����������ԭ��ClO2-�����ӷ���ʽΪ��2ClO2+2I-=2ClO2-+I2��
�ʴ�Ϊ��2ClO2+2I-=2ClO2-+I2��
����ͼʾ������pH��1��3ʱ��ClO2-��I-������I�����ɵ�I2�����ӷ���ʽΪ��ClO2-+4I-+4H+=Cl-+2I2+2H2O��
�ʴ�Ϊ��ClO2-+4I-+4H+=Cl-+2I2+2H2O��
��S2O32-��������I-��������ClO2
1 1 1
1.0��10-3 mol/L��0.01L 1.0��10-5mol
m��ClO2��=n��ClO2����M��ClO2��=1.0��10-5mol��67.5��103mg•mol-1=0.675mg
����ˮ��Ϊ 1L������ ClO2 ��Ũ��Ϊ$\frac{0.675mg}{1L}$=0.675 mg•L-1
�ʴ�Ϊ��0.675��
���� ���⿼�����Ʊ�ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ�����ע�������Ŀ������Ϣ�жϿ��ܵķ�Ӧ���Դ���д��Ӧ�ķ���ʽ�Լ����ݹ�ϵʽ���м��㣬Ϊ������״��㣬����ʱע����ᣮ

 ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�CH3CH2OH$��_{170��}^{H_{2}SO_{4}��Ũ����}$CH2�TCH2
CH2�TCH2+Br2��BrCH2CH2Br
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ�������������������Ҵ��Ƹ�1��2-���������װ����ͼ��ʾ��
�й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -130 | 9 | -116 |
��1���ڴ�װ�ø�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����d��������ȷѡ��ǰ����ĸ��
a��������Ӧ b���ӿ췴Ӧ�ٶ� c����ֹ�Ҵ��ӷ�d�����ٸ�������������
��2����װ��C��Ӧ����c����Ŀ�������շ�Ӧ�п������ɵ��������壺������ȷѡ��ǰ����ĸ��
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��3���жϸ�װ�ø���Ӧ�Ѿ�������������������ɫ��ȫ��ȥ��
��4����1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ���²㣨��ϡ������¡�����
��5�������������������������ѣ���������ķ�����ȥ��
��6����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ���DZ���������ӷ������ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ���Dz�Ʒ1��2-��������ķе�ͣ�������ȴ�����̶��������ܣ�
| A�� | ��ѧ����һ�������� | |
| B�� | ��ѧ������ʹ���Ӽ����ϣ�Ҳ����ʹԭ�Ӽ����� | |
| C�� | ��ѧ��Ӧ�����з�Ӧ�ﻯѧ�����ѣ������ﻯѧ���γ� | |
| D�� | �Ȼ��ƹ�������ˮû����������������û���ƻ���ѧ�� |
| A�� | ��ɫ��ѧ�ĺ�����Ӧ�û�ѧԭ���Ի�����Ⱦ�������� | |
| B�� | �������﷽���ѳ�������ˮ�еĵ����ף���ֹˮ�帻Ӫ���� | |
| C�� | PM 2.5��ָ������ֱ���ӽ�2.5��10-6m�Ŀ������ɢ�ڿ������γɽ��� | |
| D�� | �ߴ���㷺Ӧ����̫���ܵ�ء������оƬ���ά |
| A�� | 1��2��1��ϵ�PbO2��Pb3O4��PbO | B�� | 1��3��2��ϵ�PbO2��Pb3O4��PbO | ||
| C�� | 1��1��1��ϵ�PbO2��Pb3O4��PbO | D�� | 2��1��1��ϵ�PbO2��Pb3O4��PbO |
| A�� | ���������Ե�ǿ�� | |
| B�� | �⻯��е�ĸߵ� | |
| C�� | �⻯�ﻹԭ��ǿ�� | |
| D�� | ����������Ӧ��ˮ�������Ե�ǿ�� |