��Ŀ����
5�������λ��ͬһ���壬���ǿ����γ�������ɺ��������ƵĻ����һ������þ��Mg2B2O5•H2O����ȡ������Ĺ�������ͼ��ͼ1��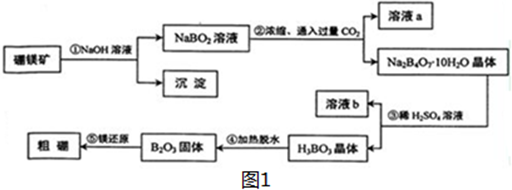
�������ͼ�ش��������⣺
��1����Һa����Һb�����ʵĻ�ѧʽ�ֱ�ΪNaHCO3��Na2SO4��
��2��д������ٵĻ�ѧ����ʽMg2B2O5•H2O+2NaOH=2NaBO2+2Mg��OH��2����
��3��д������ڵ����ӷ���ʽ2Na++4BO2-+2CO2+11H2O=Na2B4O7•10H2O��+2HCO3-��
��4��������л�ѧ��Ӧ���Է�����ԭ�������������С�����ᣬ���ϸ��ֽⷴӦ��ǿ���������ԭ������������ܽ�Ƚ�С������Һ�����������ڸ÷�Ӧ�Ľ��У�
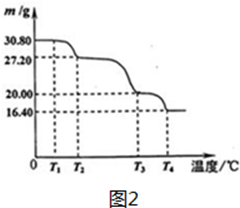
��5���������ƾ��壨NaBO3•4H2O����һ��������Ư������70�����ϼ��Ȼ���ʧȥ��
��ˮ�� ʵ���ù������ƾ�����������¶ȱ仯�������ͼ2��ʾ����T2��ʱ���þ���Ļ�ѧʽΪNaBO3•3H2O��
���� ��þ��������������Һ��Ӧ�����˳�ȥ���ʣ�NaBO2��Һ�У�ͨ������Ķ�����̼���õ�Na2B4O7•10H2O��ΪNaHCO3�����˷��룬�������������С�����ᣬ���ϸ��ֽⷴӦ��ǿ���������ԭ������������ܽ�Ƚ�С����Na2B4O7•10H2O���������ᷴӦ�õ����ᣬ��Һb�к��������ƣ����ᾧ����ȷֽ�õ�B2O3�������Mg��ԭ�õ�����
��1��������������֪���ڢٲ�ͨ�����������̼����aΪNaHCO3���ڢ۲��������ᣬbΪNa2SO4��
��2���ڢٲ�ΪMg2B2O5��H2O������������Һ��Ӧ����NaBO2��Mg��OH��2��
��3��NaBO2�Ͷ�����̼��Ӧ����Na2B4O7•10H2O��̼�����ƣ�
��4�����ϸ��ֽⷴӦ��ǿ���������ԭ����
��5���������ƾ��壨NaBO3•4H2O�������ʵ���=$\frac{30.8g}{154g/mol}$=0.2mol��T2�������仯Ϊ27.20g�������仯30.8g-27.20g=3.6g������ˮ������=$\frac{3.6g}{18g/mol}$=0.2mol���ݴ˷����жϵõ���ѧʽ��
��� �⣺��þ��������������Һ��Ӧ�����˳�ȥ���ʣ�NaBO2��Һ�У�ͨ������Ķ�����̼���õ�Na2B4O7•10H2O��ΪNaHCO3�����˷��룬�������������С�����ᣬ���ϸ��ֽⷴӦ��ǿ���������ԭ������������ܽ�Ƚ�С����Na2B4O7•10H2O���������ᷴӦ�õ����ᣬ��Һb�к��������ƣ����ᾧ����ȷֽ�õ�B2O3�������Mg��ԭ�õ�����
��1��������������֪���ڢٲ�ͨ�����������̼����aΪNaHCO3���ڢ۲��������ᣬbΪNa2SO4��
�ʴ�Ϊ��NaHCO3��Na2SO4��
��2���ڢٲ�ΪMg2B2O5��H2O������������Һ��Ӧ����NaBO2��Mg��OH��2����Ӧ����ʽΪ��Mg2B2O5•H2O+2NaOH=2NaBO2+2Mg��OH��2��
�ʴ�Ϊ��Mg2B2O5•H2O+2NaOH=2NaBO2+2Mg��OH��2��
��3��NaBO2�Ͷ�����̼��Ӧ����Na2B4O7•10H2O��̼�����ƣ����ӷ���ʽΪ2Na++4BO2-+2CO2+11H2O=Na2B4O7•10H2O��+2HCO3-���ʴ�Ϊ��2Na++4BO2-+2CO2+11H2O=Na2B4O7•10H2O��+2HCO3-��
��4��������л�ѧ��Ӧ���Է�����ԭ���ǣ����������С�����ᣬ���ϸ��ֽⷴӦ��ǿ���������ԭ������������ܽ�Ƚ�С������Һ�����������ڸ÷�Ӧ�Ľ��У�
�ʴ�Ϊ�����������С�����ᣬ���ϸ��ֽⷴӦ��ǿ���������ԭ������������ܽ�Ƚ�С������Һ�����������ڸ÷�Ӧ�Ľ��У�
��5���������ƾ��壨NaBO3•4H2O�������ʵ���=$\frac{30.8g}{154g/mol}$=0.2mol��T2��C�����仯Ϊ27.20g�������仯30.8g-27.20g=3.6g������ˮ������=$\frac{3.6g}{18g/mol}$=0.2mol����1molNaBO3•4H2Oʧȥˮ1mol����T2��ʱ���þ���Ļ�ѧʽΪNaBO3•3H2O��
�ʴ�Ϊ��NaBO3•3H2O��
���� ���⿼�黯ѧ�Ʊ�������ͼ������жϺͼ���Ӧ�ã����������ԭ���ǽ���ؼ������ضԻ�ѧ����Ŀ��飬��Ҫѧ���߱���ʵ�Ļ�����������õ��������Ѷ��еȣ�

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д�| A�� | ��ȼ�ľƾ��Ʋ�С�Ĵ��ˣ�Ӧ����������ˮ��� | |
| B�� | Ƥ��մ������Ũ��������ô���ˮ��ϴ����Ϳ��ϡ̼��������Һ | |
| C�� | ������ʵ���У�����һ��ʱ������˼����ʯ����ƿ�����������ʯ | |
| D�� | ������������������ʱ��Ϊ��ֹ�л��������ݳ�����ʹ��������ס���˳��� |
| A�� | �٢� | B�� | �ۢ� | C�� | �٢ڢۢ� | D�� | �٢ڢۢܢ� |
| A�� | ��5mL��Ͳȷ��ȡ4.55mLˮ | |
| B�� | �÷�����ƽȷ�س�ȡ6.82gʳ�� | |
| C�� | ����ʱ�ò���������©���ڵ�Һ�壬�Լ��ٹ��� | |
| D�� | ��ȡ����ʱ��Ӧѡ���л���ȡ��������ȡ�����ܶȱ����ˮ�� |
| A�� | ��˳�緽��ĵ��ݴ��� | |
| B�� | �ý��д���Һ��ë����ס�ڱ�Ѹ������ | |
| C�� | ����緽��ĸߴ��� | |
| D�� | ����������Ŀ��ý�ʪ���ޱ��º��Ŵ�������ʱ�����Ԯ |
| A�� | ������Ư���ԣ���ʹʪ�����ɫ������ɫ | |
| B�� | Ư�۵���Ч�ɷ���CaCl2��Ӧ�ܷⱣ�� | |
| C�� | ����ȥ�����л��е������Ȼ������壬�ɽ��������ͨ��ʢ��NaOH��Һ��ϴ��ƿ | |
| D�� | ����ͭ˿��������ȼ�յ�ʵ��ʱ������ƿ��Ӧ��һ��ϸɳ |
| A�� | �����£�28g C2H4��nA��̼̼˫�� | |
| B�� | 1mol��ϩ���к���˫������ĿΪNA | |
| C�� | ���³�ѹ�£�22.4L CCl4����nA��CCl4���� | |
| D�� | 100ml0.1mol/L�Ĵ�����Һ�к��е���������Ϊ0.01NA |
 ��
�� ��������2-��-2-������
��������2-��-2-������