��Ŀ����
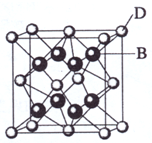 ��2009?���ϣ���֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ����ͼ��
��2009?���ϣ���֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ����ͼ����ش�
��1��AԪ�ص�������
��
��
����2��B��Ԫ�ط�����
F
F
��C��Ԫ�ط�����Cl
Cl
��B��A�γɵĻ������C ��A�γɵĻ�����е�ߣ���ԭ������������Ӽ����������Ȼ�����Ӽ�û�����
��������Ӽ����������Ȼ�����Ӽ�û�����
��3��E��Ԫ�����ڱ��е�
��
��
���ڣ���VIIB
VIIB
���Ԫ�أ���Ԫ����������
��
������+2�����ӵĵ����Ų�ʽΪ1s22s22p63s23p63d5
1s22s22p63s23p63d5
����4����ͼ�п��Կ�����D��B�γɵ����ӻ�����Ļ�ѧʽΪ
CaF2
CaF2
�������ӻ����ᄃ����ܶ�Ϊa g?cm-3�����������| 4��78g/mol |
| ag?cm-3��6.02��1023/mol |
| 4��78g/mol |
| ag?cm-3��6.02��1023/mol |
��������D��E�����ڱ���1-18����E�ŵ�7�п��ж�E�ǵ�4����VIIB���MnԪ�أ�ԭ������Ϊ25������DҲ�ڵ�4���ڣ�D��ԭ��������EС5����ԭ������Ϊ20��ӦΪCaԪ�أ�ͼ�����ӻ�����D��B�����Ӹ�����ֵΪ����8��
+6��
����8=1��2����DΪCa����B�Ļ��ϼ�Ϊ-1�ۣ�ӦΪ�ڢ�A��Ԫ�أ�B��C��ͬһ���壬B��������ǰ�棬BΪF��CΪCl��A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壬����AΪH��
������ĽǶȱȽ��⻯��ķе�ߵͣ�
����ԭ�Ӻ�������Ų��ж�Ԫ�������ڱ��е�λ�ã������������ԭ���ͺ��ع�����д���ӵĵ����Ų�ʽ��
���þ�̯�����㻯ѧʽ�����ݦ�=
���㾧���������
| 1 |
| 8 |
| 1 |
| 2 |
������ĽǶȱȽ��⻯��ķе�ߵͣ�
����ԭ�Ӻ�������Ų��ж�Ԫ�������ڱ��е�λ�ã������������ԭ���ͺ��ع�����д���ӵĵ����Ų�ʽ��
���þ�̯�����㻯ѧʽ�����ݦ�=
| m |
| V |
����⣺��D��E�����ڱ���1-18����E�ŵ�7�п��ж�E�ǵ�4����VIIB���MnԪ�أ�ԭ������Ϊ25������DҲ�ڵ�4���ڣ�D��ԭ��������EС5����ԭ������Ϊ20��ӦΪCaԪ�أ�ͼ�����ӻ�����D��B�����Ӹ�����ֵΪ����8��
+6��
����8=1��2����DΪCa����B�Ļ��ϼ�Ϊ-1�ۣ�ӦΪ�ڢ�A��Ԫ�أ�B��C��ͬһ���壬B��������ǰ�棬BΪF��CΪCl��A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壬����AΪH��
��1��AΪHԪ�أ�����Ϊ�⣬�ʴ�Ϊ���⣻
��2��BΪFԪ�أ�CΪClԪ�أ�HF�к�����������Ӽ����������ǿ���е�ϸߣ��ʴ�Ϊ��F��Cl�� ��������Ӽ����������Ȼ�����Ӽ�û�������
��3��EΪMnԪ�أ�λ�����ڱ��������ڵ����У���Ӧλ��VIIB�壬ԭ�ӵĺ�����ӵ��Ų�ʽΪ��ʧȥ2������ 1s22s22p63s23p63d54s2�����ӵĵ����Ų�ʽΪ 1s22s22p63s23p63d5��
�ʴ�Ϊ���ģ�VIIB�� �̣� 1s22s22p63s23p63d5��
��4����ͼ�п��Կ����������к���Ca�����Ӹ���Ϊ8��
+6��
=4������F�����Ӹ���Ϊ8�����߱�ֵΪ1��2��
��ѧʽΪCaF2��
��������=
=
=ag?cm-3����V=
��
�ʴ�Ϊ��CaF2��
��
| 1 |
| 8 |
| 1 |
| 2 |
��1��AΪHԪ�أ�����Ϊ�⣬�ʴ�Ϊ���⣻
��2��BΪFԪ�أ�CΪClԪ�أ�HF�к�����������Ӽ����������ǿ���е�ϸߣ��ʴ�Ϊ��F��Cl�� ��������Ӽ����������Ȼ�����Ӽ�û�������
��3��EΪMnԪ�أ�λ�����ڱ��������ڵ����У���Ӧλ��VIIB�壬ԭ�ӵĺ�����ӵ��Ų�ʽΪ��ʧȥ2������ 1s22s22p63s23p63d54s2�����ӵĵ����Ų�ʽΪ 1s22s22p63s23p63d5��
�ʴ�Ϊ���ģ�VIIB�� �̣� 1s22s22p63s23p63d5��
��4����ͼ�п��Կ����������к���Ca�����Ӹ���Ϊ8��
| 1 |
| 8 |
| 1 |
| 2 |
��ѧʽΪCaF2��
��������=
| m |
| V |
| ||
| V |
| 4��78g/mol |
| ag?cm-3��6.02��1023/mol |
�ʴ�Ϊ��CaF2��
| 4��78g/mol |
| ag?cm-3��6.02��1023/mol |
������������Ԫ���ƶ�Ϊ������������Ԫ�����ơ�λ�ü������Ų�ʽ֮�⣬��������й�Ӱ�쾧���۷е�ߵ͵�ԭ������������⣮������ۺ��Խϴ����ϸ��������ʽṹ�����ʵ�����֪ʶ��

��ϰ��ϵ�д�
�����Ŀ