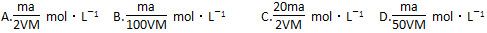��Ŀ����
��NAΪ�����ӵ�������ֵ�����������������(����)��
| A����״���£�22.4 L SO2��O2�Ļ�������к��е���ԭ����Ϊ2NA |
| B����״���£�22.4 L��ϩ�к��й��õ��ӶԵ���ĿΪ6NA |
| C���ܱ������У�3 mol H2��1 mol N2�ڸ��¡���ѹ�������������³�ַ�Ӧ�������ڵ������������Ϊ2NA |
| D��2.8 g��CO��2.8 g��N2������������Ϊ1.4NA |
��C
����

��ϰ��ϵ�д�
�����Ŀ
N0Ϊ�����ӵ�������ֵ������������ȷ����
| A��1 mol CO2�к���ԭ����N0 |
| B��1 L 1mol/L����������Һ�У�����Na����ΪN0 |
| C����״���£�11.2 Lˮ����������Ϊ0.5 N0 |
| D���ڷ�Ӧ�У�1 mol Fe���������ᷴӦʧȥ�ĵ�����Ϊ2 N0 |
��ijȨ�����ﱨ������ѧ���������з�����H3���ӣ�������ͬ���ʵ�����H3��H2�����и�����һ����ȵ��ǣ� ��
| A������ | B����� | C�������� | D��ԭ���� |
���ʵ���Ũ����ͬ��NaCl��MgCl2��AlCl3������Һ������Һ�������Ϊ3��2��1ʱ��������Һ��Cl�� �����ʵ���֮��Ϊ
| A��1��1��1 | B��1��2��3 | C��3��2��1 | D��3��4��3 |
��NAΪ�����ӵ�������ֵ������������ȷ���ǣ� ��
| A��1 mol�״��к���C��H������ĿΪ4NA |
| B��25 ��,pH=13��NaOH��Һ�к���OH-����ĿΪ0.1NA |
| C����״���£�2.24 L���麬�з��ӵ���ĿΪ0.1NA |
| D�����³�ѹ�£�Na2O2������H2O��Ӧ,������0.2 mol O2,ת�Ƶ��ӵ���ĿΪ0.4NA |
��������������غ������Ļ����Һ�У����SO42����Ũ��Ϊ0.2 mol��L��1������������0.2 mol��L��1KOH��Һʱ�����ɵij���ǡ����ȫ�ܽ⣬��Ӧ������Һ��K����Ũ��Լ��(������Һ��������H����OH��)(����)��
| A��0.125 mol��L��1 | B��0.225 mol��L��1 | C��0.250 mol��L��1 | D��0.450 mol��L��1 |
�±�Ϊ������Һ�������������������ʵ���Ũ�ȵ���ֵ�����ݱ��������ж�������Һ���ܶ���С����(����)��
| ��Һ | KOH | HNO3 | CH3COOH | HCl |
| ������������w(%) | 56 | 63 | 60 | 36.5 |
| ���ʵ���Ũ��c(mol��L��1) | 14.3 | 13.8 | 10.6 | 11.8 |
A.HCl����B��HNO3����C��CH3COOH��D��KOH
��NAΪ�����ӵ���������ֵ,������˵������ȷ����(����)
A��������,0.1 mol̼���ƾ����к���C �ĸ���Ϊ0.1NA �ĸ���Ϊ0.1NA |
| B����״����22.4 L H2O����������ΪNA |
| C��1 L 0.5 mol��L-1 CH3COOH��Һ��,CH3COO-�ĸ���Ϊ0.5NA |
| D����NO��NO2�Ļ������22.4 L��,���еĵ�ԭ����ΪNA�� |