��Ŀ����
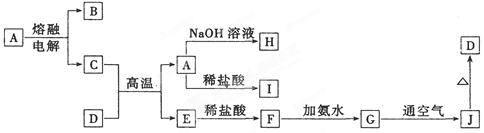
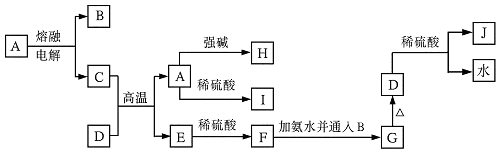
��9�֣���A��B��C��D��E��F��G��H��I�Ⱦ��ֳ������ʣ����ǵ�ת����ϵ����Ӧ����δע��������ͼ��ʾ����֪��B��C��FΪ���ʣ����³�ѹ�£�BΪ��̬��C��FΪ��̬���������н�A��EΪ��̬�������£�DΪ����ɫ��ĩ��H����ɫ��ӦΪ��ɫ��GΪ��ɫҺ�塣
��1��д��G��D��Ӧ�����ӷ���ʽ___________________________��
��2��д��B��G��Ӧ�Ļ�ѧ����ʽ_____________________________��
��3���ڢ١��ڡ��ۡ��ܡ��������Ӧ�У���H��0�ķ�Ӧ��___������ţ���
��4��E��һ�ֿ�ȼ�����壬��֪4.0gE����������ȫȼ�շų�71kJ������д���÷�Ӧ��ȼ���ȵ��Ȼ�ѧ����ʽ�� __________________________
��5����д��������A�ĵ���ʽ____________________

��1��д��G��D��Ӧ�����ӷ���ʽ___________________________��
��2��д��B��G��Ӧ�Ļ�ѧ����ʽ_____________________________��
��3���ڢ١��ڡ��ۡ��ܡ��������Ӧ�У���H��0�ķ�Ӧ��___������ţ���
��4��E��һ�ֿ�ȼ�����壬��֪4.0gE����������ȫȼ�շų�71kJ������д���÷�Ӧ��ȼ���ȵ��Ȼ�ѧ����ʽ�� __________________________
��5����д��������A�ĵ���ʽ____________________
��1�� 2Na2O2��2H2O��4Na����4OH����O2�� (2�֣�
��2��C��H2O CO��H2 (2�֣���3���ڡ��� (2�֣�
CO��H2 (2�֣���3���ڡ��� (2�֣�
(4) CO(g)��1��2O2(g)��CO2(g) ;��H=-497kJ/mol (2�֣� ��5��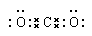
��2��C��H2O
 CO��H2 (2�֣���3���ڡ��� (2�֣�
CO��H2 (2�֣���3���ڡ��� (2�֣�(4) CO(g)��1��2O2(g)��CO2(g) ;��H=-497kJ/mol (2�֣� ��5��
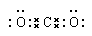
����������ͼ�⣬�ؼ�����ͻ�Ƶ㡣DΪ����ɫ��ĩ��H����ɫ��ӦΪ��ɫ��GΪ��ɫҺ�壬����D�ǹ������ƣ�G��ˮ��F����̬�������Ƶ��ʣ�E����̬���������B��̼��F��������E��CO����A��CO2��C��������H��̼�ᱵ��I���������ơ�
��3�����鳣���ķ��ȷ�Ӧ�����ȷ�Ӧ��һ�������ˮ���ᷴӦ������кͷ�Ӧ��һ��ȼ�գ���������Ϸ�Ӧ���û���Ӧ������������Ӧ��������Ƿ��ȷ�Ӧ��������ֽⷴӦ����κͼӦ��̼��������CO����ԭ���ķ�Ӧ�������ȷ�Ӧ�����Ԣڢ������ȷ�Ӧ��
��4��ȼ������ָ��һ�������£�1mol��ȼ��β��ȼ�������ȶ���������ʱ����������������CO��ȼ���ȵ��Ȼ�ѧ����ʽΪCO(g)��1��2O2(g)��CO2(g) ;��H=-497kJ/mol��
��5��CO2���ɼ��Լ��γɵĹ��ۻ��������ʽΪ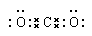 ��
��
��3�����鳣���ķ��ȷ�Ӧ�����ȷ�Ӧ��һ�������ˮ���ᷴӦ������кͷ�Ӧ��һ��ȼ�գ���������Ϸ�Ӧ���û���Ӧ������������Ӧ��������Ƿ��ȷ�Ӧ��������ֽⷴӦ����κͼӦ��̼��������CO����ԭ���ķ�Ӧ�������ȷ�Ӧ�����Ԣڢ������ȷ�Ӧ��
��4��ȼ������ָ��һ�������£�1mol��ȼ��β��ȼ�������ȶ���������ʱ����������������CO��ȼ���ȵ��Ȼ�ѧ����ʽΪCO(g)��1��2O2(g)��CO2(g) ;��H=-497kJ/mol��
��5��CO2���ɼ��Լ��γɵĹ��ۻ��������ʽΪ
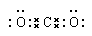 ��
��
��ϰ��ϵ�д�
 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
�����Ŀ