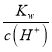��Ŀ����
����Ŀ����(Bi)λ��Ԫ�����ڱ��еڢ�A�壬���̬Ϊ��3ʱ���ȶ���������(NaBiO3)��Һ����ɫ����ȡһ������������(MnSO4)��Һ�����������εμ�������Һ����Ӧ�����������ʾ��

������ʵ�������£����н�����ȷ����
A.BiO3-��������ǿ��MnO4-
B.H2O2������������ӻ�ԭ��O2
C.H2O2���������ԣ��ܰ�KI������I2
D.��KI-������Һ�еμ���������Һ����Һһ������ɫ
���𰸡�A
��������
A. ����ɫ������(MnSO4)��Һ�м���������ɫ������(NaBiO3)��Һ����ַ�Ӧ����Һ��Ϊ�Ϻ�ɫ��˵����Ӧ������MnO4-��MnԪ�ػ��ϼ����ߣ�ʧȥ���ӱ�����ΪMnO4-������������ԭ��Ӧ�Ĺ��ɣ���������������ǿ����������������ԣ���֪BiO3-��������ǿ��MnO4-��A��ȷ��
B. �����ɫ��Һ�м������H2O2����Һ�ĺ�ɫ��ʧ���������ݣ�˵��������O2����Ԫ�ػ��ϼ����ߣ�ʧȥ���ӣ�����������H2O2�������������������O2��B����
C. ����Һ�м�������KI-������Һ����Һ������Ϊ��ɫ��������H2O2��KI����ΪI2��Ҳ������Mn2+������ʹH2O2�ֽ������O2��KI����ΪI2��I2��������Һ��Ϊ��ɫ��C����
D. �����ƾ���ǿ�������ԣ����Խ�KI����ΪI2��I2��������Һ��Ϊ��ɫ��Ҳ���Խ�KI����ΪKIO3�������Һ��һ����Ϊ��ɫ��D����
�ʺ���ѡ����A��

����Ŀ�������£���һԪ��HA����Һ��KOH��Һ�������ϣ���������仯����ʵ���������±���
ʵ���� | ��ʼŨ��/(mol��L-1) | ��Ӧ����Һ��pH | |
c(HA) | c(KOH) | ||
�� | 0.1 | 0.1 | 9 |
�� | x | 0.2 | 7 |
��1��ʵ��ٷ�Ӧ�����ҺpH=9��ԭ����___�������ӷ���ʽ��ʾ����
��2��ʵ��ٺ�ʵ�����ˮ�ĵ���̶Ƚϴ����__������Һ����ˮ�������c(OH-)=__��
��3��x__0.2(������������������=��)����x=a����������HA�ĵ���ƽ�ⳣ��Ka=__���ú�a�ı���ʽ��ʾ����
��4��������֪Ũ�ȵ�KOH�ζ�δ֪Ũ�ȵ�һԪ��HA���ζ��յ���жϷ�����__��
����Ŀ�������������������������������Ҫ�����á��ش��������⣺
(1)��֪4NH3(g)+5O2(g)=4NO(g)+6H2O(g)��H1=��alkJ/mol��4NH3(g)+6NO(g)=5N2(g)+6H2O(g)��H2=��bkJ/mol��H2O(1)=H2O(g)��H3=+ckJ/mol��д����298Kʱ������ȼ������N2���Ȼ�ѧ����ʽ___________��
(2)�����еļ��쵰��(Mb)����O2�������MbO2��Mb(aq)+O2(g)![]() MbO2(aq)������k����k���ֱ��ʾ����Ӧ���淴Ӧ�����ʳ�������V��=k����c(Mb)��P(O2)��V��=k����c(MbO2)��37��ʱ��ü��쵰�Ľ�϶�(��)��P(O2)�Ĺ�ϵ���±�[��϶�(��)ָ����O2��ϵļ��쵰��ռ�ܼ��쵰�İٷֱ�]��
MbO2(aq)������k����k���ֱ��ʾ����Ӧ���淴Ӧ�����ʳ�������V��=k����c(Mb)��P(O2)��V��=k����c(MbO2)��37��ʱ��ü��쵰�Ľ�϶�(��)��P(O2)�Ĺ�ϵ���±�[��϶�(��)ָ����O2��ϵļ��쵰��ռ�ܼ��쵰�İٷֱ�]��
P(O2) | 0.50 | 1.00 | 2.00 | 3.00 | 4.00 | 5.00 | 6.00 |
����MbO2%�� | 50.0 | 67.0 | 80.0 | 85.0 | 88.0 | 90.3 | 91.0 |
�ټ���37�桢P(O2)Ϊ2.00kPaʱ��������Ӧ��ƽ�ⳣ��K=___________��
�ڵ���ƽ��ʱ���쵰����O2�Ľ�϶�(��)��O2��ѹǿ[P(O2)]֮��Ĺ�ϵʽ��=___________(�ú���k����k����ʽ�ӱ�ʾ)��
(3)���ɼ��쵰�ĸʰ���(NH2CH2COOH)��һ���������ʣ�����Һ��������������ʽ���ڣ���ת����ϵ���£�
![]()
![]()
![]()
![]()
![]()
�ڸʰ�����Һ�м�������������ӵİٷֺ������Ĺ�ϵ��ͼ��ʾ��
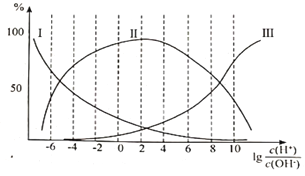
�ٴ��ʰ�����Һ��___________�ԣ�����Һ������ʱ�������ӵ�Ũ���ɴ�С��˳��Ϊ___________��
����![]() =8����Һ�м������HClʱ����Ӧ�����ӷ���ʽΪ___________��
=8����Һ�м������HClʱ����Ӧ�����ӷ���ʽΪ___________��
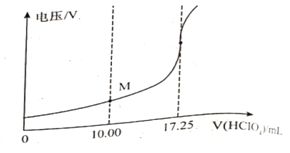
���õ�λ�ζ����ɲⶨij�ʰ�����Ʒ�Ĵ���.
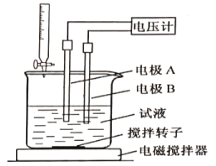
��ȡ��Ʒ150mg����һ�������£���0.1000mol/L�ĸ�������Һ�ζ�(��ʰ���1�U1������Ӧ)����õ�ѹ�仯�����HClO4��Һ�������ϵ����ͼ�����հ���ʵ�飬����HClO4��Һ�����Ϊ0.25mL������Ʒ�Ĵ���Ϊ___________%(����������һλС��)