��Ŀ����
����Ŀ������ijѧ����0.2000 mol��L��1�ı�NaOH��Һ�ζ�δ֪Ũ�����ᣬ������ɷ�Ϊ���¼�����
��������ˮϴ�Ӽ�ʽ�ζ��ܣ���ע��NaOH��Һ����0���̶�������
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
�۵���Һ������0������0���̶������£������¶���
����ȡ20.00mL����Һע���ô���Һ��ϴ������ƿ�У�������3�μ�����Һ
���ñ�Һ�ζ����յ㣬���µζ���Һ�����
��ش�
��1�����ϲ����д�����ǣ����ţ�________��
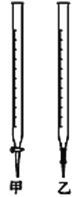
��2���ñ�NaOH��Һ�ζ�ʱ��Ӧ����NaOH��Һע��______�С�����ͼ��ѡ������������������
��3���ζ�ʱ�����ֿ��Ƶζ��ܣ�����ҡ����ƿ���۾�ע��_______________��
��4���жϵ���ζ��յ�������ǣ���ƿ����Һ_________________________��
��5�����в���������ʵ����ƫ����ǣ�______�����ţ�
A���ζ��յ�ʱ����һ�α�Һ�����ڵζ��ܼ��촦
B���۲����ʱ���ζ�ǰ���ӣ��ζ�������
C����ƿ��������ˮϴ�Ӻ�δ�ô���Һ��ϴ
D������ʽ�ζ�����ȡҺ��ʱ���ͷ�Һ��ǰ�ζ���ǰ�������ݣ�֮����ʧ
E���ζ�ʱ����ƿ����Һ�ɽ���ȥ
F�����Ʊ�NaOH��Һ����ʱ���ӹ۲�̶���
��.��6��Ϊ�˼���ij����������Ԫ�صĺ������Ƚ�������Ԥ����������Ԫ�ػ�ԭ��Fe2+������KMnO4����Һ�����������½���������ԭ�ζ���д���ζ������з�Ӧ�����ӷ���ʽ��________________________________________��KMnO4Ӧװ��___________�ζ����У�������ʽ��������ʽ�����ζ�ǰ�Ƿ�Ҫ�μ�ָʾ����___�����������������������ζ��յ���жϷ�����_____________________________��
��7��ij����CuCl2��Һ�к�������FeCl3��Ϊ�Ƶô���CuCl2��Һ���˼���______________������ҺpH��4��ʹFe3��ת��ΪFe(OH)3��������ʱ��Һ�е�c(Fe3��)��________________��[Fe(OH)3��Ksp=2.6��10-39]
���𰸡��٢� �� ��ƿ����Һ��ɫ�ı仯 �ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ ABF 5Fe2++8H++MnO4-=5Fe3++Mn2++4H2O ��ʽ �� �����һ�����Ը��������Һ��ɫ����ȥ����30 s����ɫ CuO (��Cu(OH)2��CuCO3�� Cu2(OH)2CO3) 2.6��10-9mol/L
��������
����к͵ζ�ʱ���ζ���������ˮϴ�����������ñ�Һ��ϴ����ƿ������ϴ���ζ��յ���ɫ�ı������ڲ��仯��
��ʽ�ζ���Ϊ������������ʽ�ζ����¶�Ϊ���в�������齺����
����c(����)=![]() �����������Դ���Һ�����ʵ���Ũ�ȵ�Ӱ����
�����������Դ���Һ�����ʵ���Ũ�ȵ�Ӱ����
���ݵ�ʧ�����غ㡢ԭ���غ㡢����غ���д���ӷ���ʽ��
����Fe(OH)3��Ksp= c(Fe3��) c3(OH-)������c(Fe3��)��
����1����������ˮϴ�Ӽ�ʽ�ζ��ܺ���ϴ���±�ҺŨ�ȼ�С������������ⶨ���ƫ����������������Һ��ϴ���ʢٴ������ô���Һ��ϴ��ƿ����ʹ����Һ�������ʵ������ӣ����ı�Һ��������ⶨ�������������ƿ������ϴ���ʢܴ���Ϊ���٢ܡ�
��2����������Ҫ�ü�ʽ�ζ��ܣ�ѡ���ң���Ϊ���ң�
��3���ζ�ʱ�����ֿ��Ƶζ��ܣ�����ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�仯����Ϊ����ƿ����Һ��ɫ�ı仯��
��4����Ϊ�ü�����ָʾ�����ζ��յ�ʱ������Ϊ����ƿ����Һ�ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ����Ϊ���ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
��5������c(����)=![]() �����������Դ���Һ�����ʵ���Ũ�ȵ�Ӱ����
�����������Դ���Һ�����ʵ���Ũ�ȵ�Ӱ����
A���ζ��յ�ʱ����һ�α�Һ�����ڵζ��ܼ��촦�����ı�Һ������ζ����ƫ�������⣻
B���۲����ʱ���ζ�ǰ���ӣ��ζ������ӣ����ı�Һ������ζ����ƫ�������⣻
C����ƿ��������ˮϴ�Ӻ�δ�ô���Һ��ϴ���ζ������Ӱ�죻
D������ʽ�ζ�����ȡҺ��ʱ���ͷ�Һ��ǰ�ζ���ǰ�������ݣ�֮����ʧ�����ı�Һ�����С���ζ����ƫС��
E���ζ�ʱ����ƿ����Һ�ɽ���ȥ�����ı�Һ�����С���ζ����ƫС��
F�����Ʊ�NaOH��Һ����ʱ���ӹ۲�̶��ߣ���ҺŨ�ȼ�С�����ı�Һ������ζ����ƫ�������⣻
����ABF��
��.��6���÷�Ӧ�У�Fe2+������ΪFe3+��KMnO4����ԭΪMn2+���ݵ�ʧ�����غ㡢ԭ���غ�͵���غ㣬����д�����ӷ���ʽΪ��5Fe2++8H++MnO4-=5Fe3++Mn2++4H2O������KMnO4��Һ��ǿ�����ԣ�Ӧװ����ʽ�ζ����У�����MnO4-��ԭΪMn2+�ɿ�����Һ��ɫ����ɫ��Ϊ��ɫ�����Բ���Ҫָʾ�����յ��жϿ�������MnO4-��ԭΪMn2+������Һ��ɫ����ɫ��Ϊ��ɫ����Fe2+��ȫ��Ӧ����ɫ������ȥ��
��Ϊ��5Fe2++8H++MnO4-=5Fe3++Mn2++4H2O ����ʽ�����������һ�����Ը��������Һ��ɫ����ȥ����30 s����ɫ��
��7���Ƶô���CuCl2��Һ��Ҫ��ȥ����FeCl3����ע�ⲻ�������µ����ʣ����Լ���������У�CuO (��Cu(OH)2��CuCO3�� Cu2(OH)2CO3)����ҺpH��4��c (OH-)=10-10mol/L��c(Fe3��)=![]() =
=![]() =
=![]() mol/L;
mol/L;
����CuO (��Cu(OH)2��CuCO3�� Cu2(OH)2CO3) ��2.6��10-9mol/L��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�