��Ŀ����
����Ŀ���ں����ܱ������У���10 mol CO��һ������H2S��ϼ��Ȳ��ﵽ����ƽ�⣺CO(g)��H2S(g) ![]() COS(g)��H2(g) K��1��ƽ���CO���ʵ���Ϊ8 mol������˵����ȷ����
COS(g)��H2(g) K��1��ƽ���CO���ʵ���Ϊ8 mol������˵����ȷ����
A. CO��H2S��ת����֮��Ϊ1:1
B. ��ƽ���H2S���������Ϊ4%
C. �����¶ȣ�COSŨ�ȼ�С�������÷�Ӧ�����ȷ�Ӧ
D. ��������ƽ����ϵ���ټ���CO��H2S��COS��H2��1 mol��ƽ�ⲻ�ƶ�
���𰸡�B
��������
��ӦǰCO�����ʵ���Ϊ10mol��ƽ���CO���ʵ���Ϊ8mol���跴ӦǰH2S���ʵ���Ϊn����
CO(g)��H2S(g) ![]() COS(g)��H2(g)
COS(g)��H2(g)
��ʼ��mol����10 n 0 0
�仯��mol����2 2 2 2
ƽ�⣨mol����8 n-2 2 2
���¶��¸÷�Ӧ��K=1���������ʵ�������Ũ�ȴ���ƽ�ⳣ������ʽ�����n�����ݼ���������ѧƽ���Ӱ�����ؽ����ж���
��ӦǰCO�����ʵ���Ϊ10mol��ƽ���CO���ʵ���Ϊ8mol���跴ӦǰH2S���ʵ���Ϊn����
CO(g)��H2S(g) ![]() COS(g)��H2(g)
COS(g)��H2(g)
��ʼ��mol����10 n 0 0
�仯��mol����2 2 2 2
ƽ�⣨mol����8 n-2 2 2
���¶��¸÷�Ӧ��K=1���������ݻ�ΪV����ƽ�ⳣ��K= ��
��
��ã�n=2.5������Ӧǰ��������ʵ���Ϊ2.5mol��
A�����ڷ�ӦǰCO��H2S�����ʵ����ֱ�Ϊ10mol��2.5mol��������ѧ��������ȣ���Ӧ���ĵ����ʵ�����ȣ����Զ��ߵ�ת����һ������ȣ���A����
B���÷�Ӧǰ������������ȣ���Ӧ������������ʵ������䣬��ȻΪ10mol+2.5mol=12.5mol��ƽ�����������ʵ���Ϊ��2.5-2��mol=0.5mol����ͬ������������������=���ʵ�������=0.5mol/12.5mol��100%=4.0%����B��ȷ��
C�������¶ȣ�COSŨ�ȼ�С��˵��ƽ�����������ƶ�����÷�Ӧ������ӦΪ���ȷ�Ӧ����C����
D����������ƽ����ϵ���ټ���CO��H2S��COS��H2��1 mol�����ʱ�÷�Ӧ��Ũ����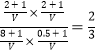 <1��˵��ƽ������������ƶ�����D������
<1��˵��ƽ������������ƶ�����D������
��ѡB��









