��Ŀ����
����Ŀ����ͼ�Dz�������Դ����������CO2ת��Ϊ���������ʵ��CO2�Ĺ̶��ʹ������Ӧ�õ�װ�á���������ʹ�õ�Li-CO2������Ϊ�ɵ缫/CO2-����LiClO4-DMSO���Һ/�Ƭ������˵����ȷ����

A. Li-CO2��ص��Һ��LiClO4��DMSO����ˮ�õ�
B. CO2�Ĺ̶�����ÿת��8mole-������3mol����
C. �������е���ת��Ϊ��ѧ��
D. ���������ɵ缫�ĵ缫��ӦʽΪ2Li2CO3+C-4e-=4Li++3CO2��
���𰸡�D
��������������ܹ���ˮ��Ӧ�����Һ������LiClO4��DMSO����ˮ�õ���A���������ݷ�Ӧ�ķ���ʽ2Li2CO3=4Li++2CO2��+O2��+4e-��֪���õ�4 mole-������2mol������̼��1mol��������ת��8mole-������6mol���壬B����ͨ��ͼʾ��֪�����Ӳ��ϵ��������������л�ѧ��ת��Ϊ���ܣ�C������ͼʾ��֪��̼��Ϊ������̼������������Ӧ�����������ɵ缫Ϊ�������缫��ӦʽΪ2Li2CO3+C-4e-=4Li++3CO2����D��ȷ����ȷѡ��D��

����Ŀ�������ڹ�ũҵ���������Ƽ���������ҪӦ�ã����й����߶�������߹㷺�о����ش�
(1)ij������ʵ�����ڳ�ʪ��ѹ��,�Ե�����Һ̬ˮΪԭ���Ʊ�����ͬʱ���������ɡ�
��֪����һ���¶Ⱥ�ѹǿ�£������ȶ��ĵ�������1mol�����ʵ���ЧӦ����Ϊ�����ʵ�������(��H)�����³�ѹ�¡�������ʵ����������±���ʾ:
���� | NH3(s) | H20(1) |
��H/ kJ��mo1-1 | -46 | -242 |
�����ϳɰ���Ӧ���Ȼ�ѧ����ʽΪ______________________��
(2)���������أ���H2��N2Ϊԭ�Ϻϳɰ���װ������ͼ��ʾ��

Q��R��Ϊ�������ݵ�ʾ�жϣ�������Ӧ�Ĵ���Ϊ___(�Q����R��)�������ĵ缫��ӦʽΪ_______________��
(3)�����ǹ�ҵ���������Ҫԭ��֮һ�������������з�������Ҫ��Ӧ���£�
I.4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g)��H=-906kJ/mol
4NO(g)+6H2O(g)��H=-906kJ/mol
II.4NH3(g)+3O2(g)![]() 2N2(g)+ 6H2O(g)��H=-126kJ/mol
2N2(g)+ 6H2O(g)��H=-126kJ/mol
���̶�����NH3��O2�Ļ��������һ������ͨ������д����ķ�Ӧ������Ӧ�������¶ȵĹ�ϵ��ͼ1��ʾ��
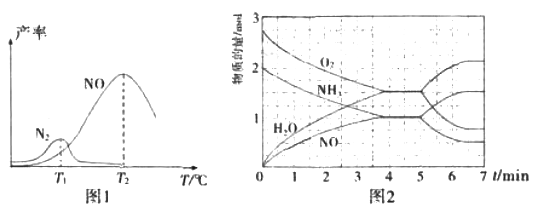
�ٴ����������У������˵��¶�Ϊ____(�T1����T2��)��
�ڵ���T1��ʱ��NO�IJ��ʽϵ͵�ԭ��Ϊ_____��
�۸���T2��ʱ��NO�IJ��ʽ��͵Ŀ���ԭ��Ϊ_____(��ѡ����ĸ)
A.�������Խ��� B.ƽ�ⳣ����С C.��Ӧ������� D.��������ˮ
��T2��(T1>T2)ʱ����20L�����ܱ������г���2molNH3��2.75mo1O2��������ӦI.��Ӧ�����и����ʵ����ʵ�������ʱ��(t)�仯��ϵ��ͼ2��ʾ��T2��ʱ���÷�Ӧ��ƽ�ⳣ��K=_____��5minʱ���ı���ijһ������������ı����������Ϊ__________��
����Ŀ��ij������ȤС������ͼװ��̽��������Na2S2O3��Һ��ϡH2SO4��Ӧ���ʵ�Ӱ�졣��ش��й����⡣
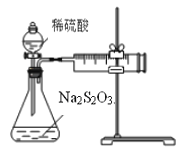
��1��д���÷�Ӧ�����ӷ���ʽ_______________________________________________________��
��2�����Ӻ�������ʼʵ��ǰ������еIJ�����_____________________________________��
��3����̽��Ũ�ȶԸ÷�Ӧ���ʣ���λmL/min����Ӱ�졣
��Ӧ�ⶨ��ʵ������Ϊ_____________��
�ڸ�ʵ��ʵʩ�����вⶨ�����������ʵ��ֵƫС��һ����Ҫԭ���ǣ�____________________��
��4������С��ͬѧ�������������ʵ�飬
ʵ�� | ��Ӧ�¶� /�� | Na2S2O3��Һ | ϡH2SO4 | H2O | |||
V/mL | c/(mol/L) | V/mL | c/(mol/L) | V/mL | |||
A | 25 | 5 | 0.1 | 10 | 0.1 | 5 | |
B | 25 | 5 | 0.2 | 5 | 0.2 | 10 | |
C | 35 | 5 | 0.1 | 10 | 0.1 | 5 | |
D | 35 | 5 | 0.2 | 5 | 0.2 | 10 | |
�� ʵ�����������˷�Ӧ��__________���ѧʽ����Ũ�ȼ�__________�����Է�Ӧ���ʵ�Ӱ�졣
�� Ԥ�ⷴӦ��������һ��ʵ�����Ϊ____________��
����Ŀ������2L�ܱ������ڣ�800��ʱ��Ӧ��2NO(g)+O2(g)![]() 2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
ʱ��(s) | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)(moll) | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
(1)��ͼ�б�ʾNO2�ı仯��������___________(����ĸ);
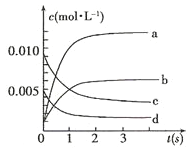
(2)800�棬��Ӧ�ﵽƽ��ʱ��NO��ת������___________��
(3)��O2��ʾ��0~2s�ڸ÷�Ӧ��ƽ������v=___________��
��һ���������İ��������(NH2COONH4)�������Ƶ��ܱ����������(��������������䣬��������������Բ���)���ں㶨�¶���ʹ��ﵽ�ֽ�ƽ�⣺NH2COONH4(s)![]() 2NH3(g)+CO2(g)��
2NH3(g)+CO2(g)��
(1)���в����жϸ÷ֽⷴӦ�Ѿ��ﵽ��ѧƽ��״̬����___________(��ѡ��);
A��2v��(NH3)=v��(CO2) B���ܱ������а��������ʵ�������
C��������CO2��NH3�����ʵ���֮�ȱ��ֲ��� D���ܱ���������ѹǿ���ֲ���
E���γ�6��N-H����ͬʱ��2��C=O������
(2)��ʹ�÷�Ӧ�ķ�Ӧ�����������___________(��ѡ��);
A����ʱ�����CO2���� B���ʵ������¶�
C����������NH2COONH4(s) D��ѡ���Ч����
(3)��ͼ��ʾ��������Ӧ�жϿ���Ӧ���л�ѧ�����յ�����___________�γ��������л�ѧ���ų�������(��д�����ڡ������ڡ���С�ڡ�)��