��Ŀ����
��ҵ�ϰ����Ĵ���������ȡ����Ļ���.��һ���¶��½�4mol NH3��4mol O2������������2L�ܱ�������,�������·�Ӧ:4NH3(g)+5O2(g) 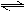 4NO(g)+6H2O(g)��
4NO(g)+6H2O(g)��
��H<0,2����ĩ������1.2molH2O,��:
(1)��H2O��ʾ�ķ�Ӧ����Ϊ________mol/(L?min).
(2)O2��2����ĩ��Ũ��Ϊ_______mol /L.
(3)�жϸ÷�Ӧ�ﵽƽ��״̬�ı�־��______(����ĸ).
a.NH3��NOŨ����� b.NO�ٷֺ������ֲ���
c.�����������ѹǿ���� d.NO������������NH3�������������
e.�����л��������ܶȱ��ֲ��� f.O2���������ٸı�
(4)�����NH3��ת����,���д�ʩ���е��� (����ĸ).
a.��װ�����ٳ���O2 b.�ı����
c.����ѹǿ d.����¶�
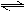 4NO(g)+6H2O(g)��
4NO(g)+6H2O(g)����H<0,2����ĩ������1.2molH2O,��:
(1)��H2O��ʾ�ķ�Ӧ����Ϊ________mol/(L?min).
(2)O2��2����ĩ��Ũ��Ϊ_______mol /L.
(3)�жϸ÷�Ӧ�ﵽƽ��״̬�ı�־��______(����ĸ).
a.NH3��NOŨ����� b.NO�ٷֺ������ֲ���
c.�����������ѹǿ���� d.NO������������NH3�������������
e.�����л��������ܶȱ��ֲ��� f.O2���������ٸı�
(4)�����NH3��ת����,���д�ʩ���е��� (����ĸ).
a.��װ�����ٳ���O2 b.�ı����
c.����ѹǿ d.����¶�
 ��3��bcf ��4��a
��3��bcf ��4��a��

��ϰ��ϵ�д�
�����Ŀ
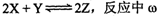 ��Z�����ʵ������������¶�T�ı仯����ͼ��ʾ�������ж���ȷ���ǣ� ��
��Z�����ʵ������������¶�T�ı仯����ͼ��ʾ�������ж���ȷ���ǣ� ��
 2SO3(g) ����H<0������Ӧ������ʱ��ı仯������Ը��ݴ������ж�����˵��������ȷ����
2SO3(g) ����H<0������Ӧ������ʱ��ı仯������Ը��ݴ������ж�����˵��������ȷ����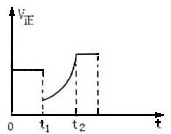
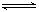 2C��2D(�����ʾ�Ϊ����)�ڲ�ͬ����²��������4 �ַ�Ӧ���ʣ����з�Ӧ������
2C��2D(�����ʾ�Ϊ����)�ڲ�ͬ����²��������4 �ַ�Ӧ���ʣ����з�Ӧ������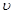 ��C����0.8mol / (L��s)
��C����0.8mol / (L��s) nZ(g)��2W(g), 5 minĩ������0.3 mol W������֪��ZŨ�ȱ仯����ʾ�ķ�Ӧƽ������Ϊ0.03 mol��L��1��min��1����������Ӧ��Z����ķ�Ӧ����ʽϵ��n��ֵ��(��)
nZ(g)��2W(g), 5 minĩ������0.3 mol W������֪��ZŨ�ȱ仯����ʾ�ķ�Ӧƽ������Ϊ0.03 mol��L��1��min��1����������Ӧ��Z����ķ�Ӧ����ʽϵ��n��ֵ��(��) cC(g)����H��0���ڵ��������½��С��ı�������Ӧ�������ڢ����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
cC(g)����H��0���ڵ��������½��С��ı�������Ӧ�������ڢ����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��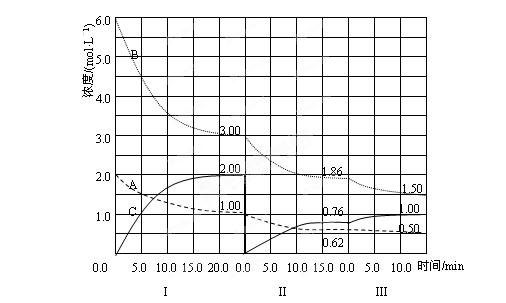
 �ķ�����_________,��ȡ�Ĵ�ʩ��________;
�ķ�����_________,��ȡ�Ĵ�ʩ��________; �͵ڢ�η�Ӧ�¶�(T3)�ĸߵͣ�T2 T3(�>����<����=�������жϵ�������_________________________________________;
�͵ڢ�η�Ӧ�¶�(T3)�ĸߵͣ�T2 T3(�>����<����=�������жϵ�������_________________________________________;  ��ʱ��仯�����ƣ������ϱ�����A��B��C����
��ʱ��仯�����ƣ������ϱ�����A��B��C����
 4NO+6H2O(g)�������Dz�ͬ����µķ�Ӧ����
4NO+6H2O(g)�������Dz�ͬ����µķ�Ӧ���� ������������ ( )
������������ ( )