��Ŀ����
13��ij��ȤС����ʵ������ͭ������Ϊԭ�ϣ����ö��ַ�����ȡ����ͭ���Ʊ��������£�����һ
��1����ͬѧȡ6.4gͭƬ��10mL 18mol•L-1Ũ���ᣬ�����Թ��й���ʱ���֣�ͭ���ȵ�Ũ���ᷴӦ��û�еõ�Ԥ�ڵ���ɫ��Һ���������Թܵײ�������ɫ��������ͬѧΪ����֤���а�ɫ��������Ҫ�ɷ֣��������ʵ�飮
ʵ�鲽�裺�㵹���ϲ�Һ��������ð�ɫ�Ĺ����м�����������ˮ���ӱ߽��裮
ʵ������ɫ�����ܽ⣬��Һ��Ϊ��ɫ��
ʵ����ۣ����ð�ɫ����Ļ�ѧʽΪ_CuSO4��
��2����ͬѧ���ͬѧ����ͬ��ʵ�飬���۲쵽���ȹ����У��Թ��ڱ��ϲ�������������ɫ�������ʣ��������ȣ�����ɫ��������������������Ũ�������ʧ��ͬʱ������ʹƷ����Һ��ɫ�����壮����ɫ������ʧ��ԭ���ǣ��û�ѧ��Ӧ����ʽ�ش�_S+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$3SO2��+2H2O��ֱ�����Ӧ��ϣ������Թ��л���ͭƬʣ�࣮
������
��3����ͬѧ��Ϊ����Ƶ�ʵ�鷽�����ã����Լ���Ƶ�˼·�ǣ�2Cu+O2$\frac{\underline{\;\;��\;\;}}{\;}$2CuO��CuO+H2SO4�TCuSO4+H2O��
�Աȼķ���������Ϊ��ͬѧ���ŵ��Ǣٲ�������������ͭ�����ĵ�������٣��ڲ�������Ⱦ��SO2��
������
��4����ͬѧȡһͭƬ��ϡ��������Թ��У��������е���˫��ˮ��������Һ����ɫ��д����Ӧ�Ļ�ѧ��Ӧ����ʽCu+H2O2+H2SO4�TCuSO4+2H2O��
���� ��1������ͭ��ĩΪ��ɫ���Դ���������
��2���ɴ��ڵ�Ԫ�ؿ�֪����ɫ��������ΪS��S��Ũ���ᷢ��������ԭ��Ӧ��ʹ����ʧ��
��3���ù����в������ж������������
��4��Cu����ԭ����˫��ˮ��������������������ԭ��Ӧ��������ͭ��
��� �⣺��1����Ϊ����ͭ��ĩΪ��ɫ�����ԻҰ�ɫ�Ĺ����м�����������ˮ�����۲쵽��ҺΪ��ɫ�������ð�ɫ����ΪCuSO4��
�ʴ�Ϊ��CuSO4��
��2���Թ��ڱ��ϲ�������������ɫ�������ʣ��ɴ��ڵ�Ԫ�ؿ�֪����ɫ��������ΪS��S���л�ԭ�ԣ�Ũ�������ǿ�����ԣ�
S��Ũ���ᷢ��������ԭ��ӦΪS+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$3SO2��+2H2O����ʹ����ɫ������ʧ��
�ʴ�Ϊ��S+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$3SO2��+2H2O��
��3����2Cu+O2$\frac{\underline{\;\;��\;\;}}{\;}$2CuO��CuO+H2SO4=CuSO4+H2O���Աȷ�Ӧ���̷�����֪����ͬѧ��Ƶ��Ʊ����̣����ɵ���������ͭ�����������٣���Ӧ����������������Ⱦ�������ɣ�
�ʴ�Ϊ���ٲ�������������ͭ�����ĵ�������٣��ڲ�������Ⱦ��SO2��
��4��ͭƬ��ϡ��������Թ��У��������е���˫��ˮ��������Һ����ɫ���Ƿ�����������ԭ��Ӧ���£��÷�ӦΪCu+H2O2+H2SO4�TCuSO4+2H2O��
�ʴ�Ϊ��Cu+H2O2+H2SO4�TCuSO4+2H2O��
���� ���⿼��Ũ��������ʣ���ȷŨ�������ǿ�����Լ������Ļ�ѧ��Ӧ�ǽ����Ĺؼ����ѶȲ���

| A�� | һ���¶Ⱥ�ѹǿ�£�������̬��������Ĵ�С�ɹ�������ķ��Ӵ�С���� | |
| B�� | һ���¶Ⱥ�ѹǿ�£�������̬��������Ĵ�С�ɹ�������ķ��������� | |
| C�� | ��ͬ�����壬�������ͬ�������������ķ�����Ҳ��ͬ | |
| D�� | ���³�ѹ�£���ͬ��������CuO��H2O��O2�������ͬ |
 25��ʱ����һ�����ı����ᣨ����ˮ���ᣩ��ˮϡ�ͣ�ϡ��������Һ�ĵ����Ա仯��ͼ��ʾ��������˵����ȷ���ǣ�������
25��ʱ����һ�����ı����ᣨ����ˮ���ᣩ��ˮϡ�ͣ�ϡ��������Һ�ĵ����Ա仯��ͼ��ʾ��������˵����ȷ���ǣ�������| A�� | ����ĵ���ƽ�ⳣ����a��b��c | |
| B�� | ��Һ��n��H+����b��a��c | |
| C�� | a��b��c�������Һ���У�c��H+��=c��CH3COO-��+c��OH-�� | |
| D�� | ��c�㵽b�㣬����ĵ���Ȳ������� |

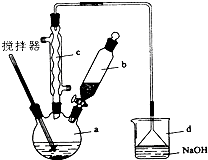 ʵ�����Ʊ�����ͪ�Ļ�ѧ����ʽΪ��
ʵ�����Ʊ�����ͪ�Ļ�ѧ����ʽΪ��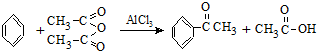
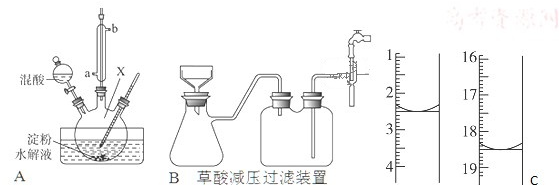
 ��
��