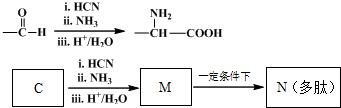
��Ŀ����
��������V���ڲ��Ͽ�ѧ������Ҫ���ã�����Ϊ���Ͻ��ά���ء��������Dzⶨ��ʯ�з��ĺ�����������Ӧ��
��1����ϡ�����ܽ��������������õ���VO2��2SO4��Һ��д���÷�Ӧ�Ļ�ѧ����ʽ ���÷�Ӧ ��ѡ��ǡ����ǡ���������ԭ��Ӧ��
��2������֪Ũ�ȵ������ữ��H2C2O4��H2C2O4��CΪ+3�ۣ���Һ���ζ���VO2��2SO4��Һ������������ӷ���ʽ����д���ʵĻ�ѧʽ�����ӷ��ţ�
VO2++ H2C2O4+ �� VO2++ CO2+
��3����Ӧ�������������� ������ԭ��Ԫ���� ��
��4����֪�÷�Ӧ�ܷ��������ữ������Ҫ֪������ ��Դ�С��
��1����ϡ�����ܽ��������������õ���VO2��2SO4��Һ��д���÷�Ӧ�Ļ�ѧ����ʽ
��2������֪Ũ�ȵ������ữ��H2C2O4��H2C2O4��CΪ+3�ۣ���Һ���ζ���VO2��2SO4��Һ������������ӷ���ʽ����д���ʵĻ�ѧʽ�����ӷ��ţ�
��3����Ӧ��������������
��4����֪�÷�Ӧ�ܷ��������ữ������Ҫ֪������
���㣺������ԭ��Ӧ
ר�⣺������ԭ��Ӧר��
��������1����ϡ�����ܽ��������������õ���VO2��2SO4��Һ������ʽΪ���������ᷴӦ����Ӧ����ʽΪ��V2O5+H2SO4�T��VO2��2SO4+H2O����VO2+��VԪ�ػ��ϼ���+5�õ���VO2��2SO4��Һ��VԪ�ػ��ϼ���+5��˵���÷�Ӧ�Ƿ�������ԭ��Ӧ��
��2��VO2+��VԪ�ػ��ϼ���+5�۽���ΪVO+��+3�ۣ�H2C2O4��̼Ԫ����+3������ΪCO2��+4�ۣ�H2C2O4�ǻ�ԭ�������ϼ���������С������Ϊ2������VO2+ϵ��Ϊ1��H2C2O4ϵ��Ϊ1���ٸ���ԭ���غ㣨����غ㣩��ƽ�������ʵ�ϵ�����õ����Ż�ԭ����ʧ���ӵ�Ԫ��ָ��õ��ӵ�Ԫ�أ�
��3���ɣ�2����֪CԪ�صĻ��ϼ����ߣ�VԪ�صĻ��ϼ۽��ͣ�
��4�������ữҪ�������᱾���ĸ�-���ȵĻ�ԭ�ԣ�
��2��VO2+��VԪ�ػ��ϼ���+5�۽���ΪVO+��+3�ۣ�H2C2O4��̼Ԫ����+3������ΪCO2��+4�ۣ�H2C2O4�ǻ�ԭ�������ϼ���������С������Ϊ2������VO2+ϵ��Ϊ1��H2C2O4ϵ��Ϊ1���ٸ���ԭ���غ㣨����غ㣩��ƽ�������ʵ�ϵ�����õ����Ż�ԭ����ʧ���ӵ�Ԫ��ָ��õ��ӵ�Ԫ�أ�
��3���ɣ�2����֪CԪ�صĻ��ϼ����ߣ�VԪ�صĻ��ϼ۽��ͣ�
��4�������ữҪ�������᱾���ĸ�-���ȵĻ�ԭ�ԣ�
���
�⣺��1����ϡ�����ܽ��������������õ���VO2��2SO4��Һ������ʽΪ���������ᷴӦ����Ӧ����ʽΪ��V2O5+H2SO4�T��VO2��2SO4+H2O����VO2+��VԪ�ػ��ϼ���+5�õ���VO2��2SO4��Һ��VԪ�ػ��ϼ���+5��˵���÷�Ӧ�Ƿ�������ԭ��Ӧ��
�ʴ�Ϊ��V2O5+H2SO4�T��VO2��2SO4+H2O�����ǣ�
��2��VO2+��VԪ�ػ��ϼ���+5�۽���ΪVO2+��+4�ۣ�H2C2O4��̼Ԫ����+3������ΪCO2��+4�ۣ�H2C2O4�ǻ�ԭ�������ϼ���������С������Ϊ2������VO2+ϵ��Ϊ2��H2C2O4ϵ��Ϊ1������ԭ���غ��֪��VO2+��ϵ��Ϊ2��CO2ϵ��Ϊ2��H2Oϵ��Ϊ2��Ȼ��ͨ������غ㣬�ÿյ�һ���������ӣ���ƽ�����ӷ���ʽΪ2VO2++H2C2O4+2H+�TVO2++2CO2+2H2O���ʴ�Ϊ��2��1��2H+��2��2��2H2O��
��3���ɣ�2����֪CԪ�صĻ��ϼ����ߣ�VԪ�صĻ��ϼ۽��ͣ���VO2+Ϊ������������ԭ��Ԫ��Ϊ+5�۵�V���ʴ�Ϊ��VO2+��+5�۵�V��
��4����֪�÷�Ӧ�ܷ��������ữ��Ҫ�������᱾���ĸ�-���ȵĻ�ԭ�ԣ�����Ҫ֪��VO2+�����������º����������Ե���Դ�С���ʴ�Ϊ��VO2+�����������º����������ԣ�
�ʴ�Ϊ��V2O5+H2SO4�T��VO2��2SO4+H2O�����ǣ�
��2��VO2+��VԪ�ػ��ϼ���+5�۽���ΪVO2+��+4�ۣ�H2C2O4��̼Ԫ����+3������ΪCO2��+4�ۣ�H2C2O4�ǻ�ԭ�������ϼ���������С������Ϊ2������VO2+ϵ��Ϊ2��H2C2O4ϵ��Ϊ1������ԭ���غ��֪��VO2+��ϵ��Ϊ2��CO2ϵ��Ϊ2��H2Oϵ��Ϊ2��Ȼ��ͨ������غ㣬�ÿյ�һ���������ӣ���ƽ�����ӷ���ʽΪ2VO2++H2C2O4+2H+�TVO2++2CO2+2H2O���ʴ�Ϊ��2��1��2H+��2��2��2H2O��
��3���ɣ�2����֪CԪ�صĻ��ϼ����ߣ�VԪ�صĻ��ϼ۽��ͣ���VO2+Ϊ������������ԭ��Ԫ��Ϊ+5�۵�V���ʴ�Ϊ��VO2+��+5�۵�V��
��4����֪�÷�Ӧ�ܷ��������ữ��Ҫ�������᱾���ĸ�-���ȵĻ�ԭ�ԣ�����Ҫ֪��VO2+�����������º����������Ե���Դ�С���ʴ�Ϊ��VO2+�����������º����������ԣ�
���������⿼��������ԭ��Ӧ��Ϊ��Ƶ���㣬����������ԭ��Ӧ��ƽ��������������ԵıȽ�Ϊ���Ĺؼ���ע��������Ϣ�������ע��֪ʶǨ��Ӧ�������Ŀ��飬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
���з�Ӧ�����ӷ���ʽ����ȷ���ǣ�������
| A����Ư����ͨ��������SO2���壺Ca2++2ClO-+SO2+H2O�TCaSO3��+2HClO | ||||
| B����CO2ͨ�뵽�������Һ�У�2Na++CO32-+CO2+H2O�T2NaHCO3�� | ||||
| C����Ba��OH��2��Һ�м���������NaHCO3��Һ��Ba2++2OH-+2HCO3-�TBaCO3��+CO32-+2H2O | ||||
D��������FeCl3��Һ�����ˮ���ƽ��壺Fe3++3H2O
|
����˵���У���ȷ���ǣ�������
| A�����ڵ�ԭ��֮�������ý�����ѧ�� |
| B�������ͷǽ���Ԫ�ص�����ϣ������γ����Ӽ� |
| C���ǽ���Ԫ�ص�ԭ�Ӱ뾶�������Ӱ뾶С������⣩������Ԫ�ص�ԭ�ӵ�ԭ�Ӱ뾶�������Ӱ뾶�� |
| D����Ԫ��ԭ�Ӱ뾶����Ԫ��ԭ�Ӱ뾶��������Ӱ뾶һ�����ҵ�ԭ�Ӱ뾶�� |
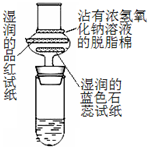 Ũ���������Ƿ�Ӧ����ʾʵ��װ�������£�ʵ��ʱ�ڴ��Թ����ȷ���2gϸС���������ǣ��μ�2-3��̼����Ũ��Һ���ټ���1.5mL 98%��Ũ���ᣬѸ������������ܵĵ�����Ƥ�������Կ���������Ѹ�ٱ�ڣ�����������ͣ��γɶ����״��������--���������������ʵ��Ч���������Ե�ԭ��
Ũ���������Ƿ�Ӧ����ʾʵ��װ�������£�ʵ��ʱ�ڴ��Թ����ȷ���2gϸС���������ǣ��μ�2-3��̼����Ũ��Һ���ټ���1.5mL 98%��Ũ���ᣬѸ������������ܵĵ�����Ƥ�������Կ���������Ѹ�ٱ�ڣ�����������ͣ��γɶ����״��������--���������������ʵ��Ч���������Ե�ԭ��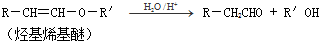
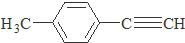 ����һ��·�����£�
����һ��·�����£�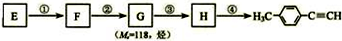
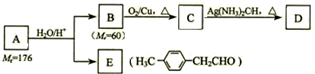
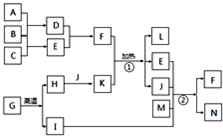 ���¿�ͼ�е���ĸ����1-20��Ԫ���γɵij������ʼ��������Щ����֮��ת����ϵ��ͼ��ʾ������A��B��CΪ���ʣ�����Ϊ�����J�����³�Һ̬��B��A��ȼ�ղ�����ɫ���棬ʵ���ҳ��âٷ�Ӧ��ȡ����E����Ӧ��Ϊ��°��Ƽ����Ҫ��Ӧԭ����LΪ�����ĸ������
���¿�ͼ�е���ĸ����1-20��Ԫ���γɵij������ʼ��������Щ����֮��ת����ϵ��ͼ��ʾ������A��B��CΪ���ʣ�����Ϊ�����J�����³�Һ̬��B��A��ȼ�ղ�����ɫ���棬ʵ���ҳ��âٷ�Ӧ��ȡ����E����Ӧ��Ϊ��°��Ƽ����Ҫ��Ӧԭ����LΪ�����ĸ������


