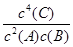��Ŀ����
��ѧƽ�ⳣ��(K)�����볣��(Ka��Kb)���ܶȻ�����(Ksp)�ȳ����DZ�ʾ���ж��������ʵ���Ҫ���������й�����Щ������˵���У���ȷ����
| A����ѧƽ�ⳣ���Ĵ�С���¶ȡ�Ũ�ȡ�ѹǿ�йأ�������� |
| B��Ka(HCN)<Ka(CH3COOH)˵����ͬ���ʵ���Ũ��ʱ������������Աȴ���ǿ |
| C�����Ȼ�����Һ�м���ͬŨ�ȵ�̼���ƺ���������Һ���Ȳ���BaSO4��������Ksp(BaSO4)>Ksp(BaCO3) |
| D�����¶�����ʱ�����ᡢ����ĵ��볣��(Ka��Kb)��� |
D
����

2010��ŵ������ѧ����ѧ��������¡��տ˵���λ��ѧ�ң��Ա��������ڡ��ٴ�����ż����������о���������ڴ�����˵����ȷ���ǣ� ��
| A������ֻ�ı䷴Ӧ������Ӧ���� |
| B������ͨ�����߷�Ӧ�Ļ�����ӿ췴Ӧ���� |
| C�������ܹ��ı䷴Ӧ�ķ�Ӧ�� |
| D���������ܸı䷴Ӧ���ת���� |
����Ⱥ����ܱ�������ͨ��SO2��NO2��һ��������ʹ��ӦSO2(g)��NO2(g)  SO3(g)��NO(g)�ﵽƽ�⣬����Ӧ������ʱ��仯��ʾ��ͼ��ͼ��ʾ����ͼ�ɵó�����ȷ�����ǣ� ��
SO3(g)��NO(g)�ﵽƽ�⣬����Ӧ������ʱ��仯��ʾ��ͼ��ͼ��ʾ����ͼ�ɵó�����ȷ�����ǣ� ��
| A����Ӧ��c��ﵽƽ��״̬ |
| B����Ӧ��Ũ�ȣ�a��С��b�� |
| C����Ӧ�������������������������� |
| D����t1����t2ʱ��SO2��ת���ʣ�a��b��С��b��c�� |
��֪(CH3COOH)2(g)  2CH3COOH(g)����ʵ���ò�ͬѹǿ�£���ϵ��ƽ��Ħ������
2CH3COOH(g)����ʵ���ò�ͬѹǿ�£���ϵ��ƽ��Ħ������ ���¶�(T)�ı仯������ͼ��ʾ������˵����ȷ����(����)
���¶�(T)�ı仯������ͼ��ʾ������˵����ȷ����(����)
| A���ù��̵Ħ�H<0 |
| B������ѹǿ��p(a)<p(b)��p(c) |
| C��ƽ�ⳣ����K(a)��K(b)<K(c) |
| D���ⶨ�������Է�������Ҫ�ڸ�ѹ���������� |
��һ�㶨�������г���2molA��1molB������Ӧ��2A(g)+ B(g) xC(g)���ﵽƽ���C���������Ϊ��%����ά���������ݻ����¶Ȳ��䣬����ʼ���ʵ���A��0.6mol��B��0.3mol��C��1.4mol�����������ﵽƽ���C�����������Ϊ��%����xֵΪ
xC(g)���ﵽƽ���C���������Ϊ��%����ά���������ݻ����¶Ȳ��䣬����ʼ���ʵ���A��0.6mol��B��0.3mol��C��1.4mol�����������ﵽƽ���C�����������Ϊ��%����xֵΪ
| A��ֻ��Ϊ2 | B��ֻ��Ϊ3 |
| C��������2��Ҳ������3 | D����ȷ�� |
��N2��H2�Ļ������ֱ����ס��ҡ������������н��кϳɰ���Ӧ������һ��ʱ���Ӧ����Ϊ��
v��(H2)��3 mol��L��1��min��1��
v��(N2)��2 mol��L��1��min��1��
v��(NH3)��1 mol��L��1��min��1��
���ʱ�������������кϳɰ��ķ�Ӧ���ʵĴ�С��ϵΪ(����)
| A��v��>v��>v�������������� | B��v��>v��>v�� |
| C��v��>v��>v�� | D��v����v����v�� |
�ں����ܱ�������ͨ��A��B�������壬��һ�������·�����Ӧ��
2A(g)��B(g)  2C(g)����H>0��
2C(g)����H>0��
�ﵽƽ��ı�һ������(x)��������(y)һ������ͼ�����ߵ���(����)
| ѡ�� | x | y |
| A | ��ͨ��A | B��ת���� |
| B | ������� | A��������� |
| C | ѹǿ | �������������ʵ��� |
| D | �¶� | �������������ʵ��� |
���и����ȷ����
| A�������ʵ���Ũ�ȵ�������Һ�Т� NH4Al(SO4)2�� NH4Cl����CH3COONH4���� NH3��H2O�� c(NH4+)���ɴ�С��˳���Ǣ�>��>��>�� |
B�������£���0��01mol/L NH4HSO4��Һ�еμ�NaOH��Һ������ |
| C��25��ʱ��0��1mol/LCH3COOH��ҺV1 mL��0��1mol/L NaOH��ҺV2mL��ϣ���V1��V2��������Һ��pHһ��С��7 |
D�����ڷ�Ӧ �����κ��¶��¶����Է����� �����κ��¶��¶����Է����� |
 4C(g)����ƽ��ʱc(A)=1.00mol?L-1���ֽ�ѹǿ��С��1.01��105Pa������ƽ���c(A)=0.18mol?L-1��������˵����ȷ���� ( )
4C(g)����ƽ��ʱc(A)=1.00mol?L-1���ֽ�ѹǿ��С��1.01��105Pa������ƽ���c(A)=0.18mol?L-1��������˵����ȷ���� ( )