��Ŀ����
��ѧ������Ӧ�Ļ�ѧ����ʽ��A��B��X��Y��H2O��H2O�� A��B��X��Y��δ��ƽ����Ӧ������ȥ���������������漰��Ӧʽ�е�H2O�Ѿ���ȥ����ش�
��1�������£���AΪ����ɫ���壬BΪ�ǽ��������A��B����ʹƷ����Һ��ɫ��A��B��X��Y������Է�������X��Y����÷�Ӧ�Ļ�ѧ����ʽΪ________________������Y�������ӵ��Լ���________________��
��2����Y�ǻ���ɫ���壬��A��B�����ʵ���֮��Ϊ1��4���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��3����AΪ�����Ľ������ʣ�YΪ��̬���ʣ�B��Һ��ǿ���Ի�ǿ����ʱ��A��B��Ӧ���ܽ��С�д��A��B��Ӧ�����ӷ���ʽ��_________________��_________________��
��4����AΪ�ǽ������ʣ���������ԭ�Ӻ��������������Ǵ�����������2����B����ҺΪijŨ�ᣬ����A��B�����ʵ���֮��Ϊ1��4����Ӧ���������뻹ԭ�������ʵ���֮���� ��
��5����A��B��Ϊ�����YΪ��ɫ��������������Ԫ�أ���BΪ��������ЧӦ����Ҫ���壬��Bͨ��ij��Һ�������Y��д��Y���ܵĻ�ѧʽ______________��д������Y��һ�����ӷ���ʽ______________________________��
��1��Cl2+SO2+2H2O=2HCl+H2SO4 AgNO3��ϡ����
��2��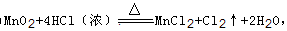
��3��2Al+6H+�T2Al3++3H2����2Al+2OH-+2H2O�T2AlO2-+3H2��
��4��4��1
��5��H2O+CO2+SiO32-=H2SiO3��+CO32-��H2O+2CO2+SiO32-=H2SiO3��+2HCO3-
���������������1��AΪ����ɫ���壬ӦΪCl2��BΪ�ǽ��������A��B����ʹƷ����Һ��ɫ����BΪSO2�����߷�Ӧ������������ᣬA+B��X+Y������Է�������X��Y����XΪH2SO4��YΪHCl����Ӧ�Ļ�ѧ����ʽΪCl2+SO2+2H2O=2HCl+H2SO4�����������ӣ��ɼ��������ữ�������������ɵ��Ȼ������������ᣬ
��2��Y�ǻ���ɫ���壬ӦΪCl2����A��B�����ʵ���֮��Ϊ1��4����A��B�ķ�ӦӦΪŨ����Ͷ������̵ķ�Ӧ������ʵ�����Ʊ���������Ӧ�Ļ�ѧ����ʽΪ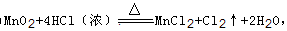
��3����AΪ�����Ľ������ʣ�YΪ��̬���ʣ�B��Һ��ǿ���Ի�ǿ����ʱ��A��B��Ӧ���ܽ��У���AΪAl��BΪǿ��������������Һ��YΪH2��A��B��Ӧ�����ӷ���ʽΪ2Al+6H+�T2Al3++3H2����2Al+2OH-+2H2O�T2AlO2-+3H2����
��4����AΪ�ǽ������ʣ���������ԭ�Ӻ��������������Ǵ�����������2����ӦΪCԪ�أ�B����ҺΪijŨ�ᣬ����A��B�����ʵ���֮��Ϊ1��4��ӦΪC��Ũ����ķ�Ӧ����Ӧ�ķ���ʽΪC+4HNO3=CO2��+4NO2��+2H2O����Ӧ���������뻹ԭ�������ʵ���֮����4��1���ʴ�Ϊ��4��1��
��5��YΪ��ɫ��������������Ԫ�أ���ӦΪH2SiO3��BΪ��������ЧӦ����Ҫ���壬ӦΪCO2��A��B�ķ�ӦӦΪ�����Թ����κͶ�����̼�ķ�Ӧ����Ӧ�����ӷ���ʽΪH2O+CO2+SiO32-=H2SiO3��+CO32-��H2O+2CO2+SiO32-=H2SiO3��+2HCO3-��
���㣺����������ƶϵ����֪ʶ��

���з�Ӧ�У���Ӧ������������ص���
| A���Ҵ�����ͨ�����ȵ�CuO��ĩ | B��������̼ͨ��Na2O2��ĩ |
| C������V2O5�������ȷ�Ӧ | D����п��Ͷ��Cu(NO3)2��Һ |
����������ת��;����ijЩ��Ӧ�����Ͳ�����ʡ�ԣ������й�˵����ȷ���ǡ����� 
| A����;���ٺ͢ڷֱ���ȡ1molFeCl2�������ϸ�����1molFe����ת��2mole- |
| B����;���ۺֱܷ͢���ȡ1 mol Na2SO4�������ϸ�����1 mol Cl2����ת��2mol e- |
| C����;���ݺֱ͢���ȡ1 mol H2SO4�������ϸ�����1mol S����ת��6mol e- |
| D������˵��������ȷ |
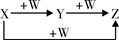
�ס��ҡ����������������У��ס��ҡ�����������ͬ��ij��Ԫ�أ�����֮���������ת����ϵ��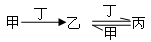 �������й����ʵ��ƶϲ���ȷ����
�������й����ʵ��ƶϲ���ȷ����
| A������Ϊ��̿��������O2 | B������ΪSO2�������ǰ�ˮ |
| C������ΪFe������������ | D������ΪNaOH��������CO2 |
��ȥ�ܷ�ʳƷ��װ���ڵ��������ӳ�ʳƷ�ı����ڣ����д�ʩ�����ӳ�ʳƷ�����ڵ���
| A���ʵ����Ӻ�����ṹ��Ԫ����Ȼ������ | B����ʳ��䵪��װ |
| C������װ�����۵�����Сֽ�� | D������װ����ʯ������Сֽ�� |
���л�ѧʵ�顢�۲�ʵ������ͨ�������������ó���ȷ�Ľ����ǻ�ѧѧϰ�ķ���֮һ�����ж��й�ʵ����ʵ�Ľ�����ȷ����(����)
| A����N2������O2�ڸ��»�ŵ�������·�Ӧ�õ�����ɫ�����壬˵��NOΪ����ɫ���� |
| B���øɾ��IJ�˿պȡ������Һ�ھƾ������������գ����ֻ���ʻ�ɫ������Һ�к�Na�� |
| C����AgNO3����Һ�м���п�ۣ����û�������˵��Ag���Ļ�ԭ�Ա�Zn2��ǿ |
| D��Ũ������������У���ʹ����̿����˵��Ũ���������ˮ�� |
�����εĹ�������ټ���ʱ������������ڼ�ˮ�ܽ�ʱ�г������ɣ��ҳ�������ϡ���ᡣ�������������Ļ������
| A��BaCl2��(NH4)2SO4 | B��AgNO3��NH4Cl |
| C��FeCl3��NaHCO3 | D��KCl��Na2CO3 |
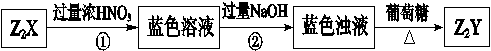
 ��X��W������
��X��W������