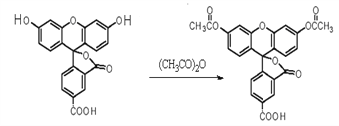
��Ŀ����
����Ŀ����1��31Ga��̬ԭ�ӵĺ�������Ų�ʽ��___________��C��N��O����Ԫ�ص�һ�����ܴӴ�С��˳����____________��д��һ���� OH- ��Ϊ�ȵ�����ķ���Ϊ_________________(�ѧʽ)��
��2����(N2H4)���ӿ���ΪNH3�����е�һ����ԭ�ӱ���NH2��������ȡ���γɵ���һ�ֵ����⻯�
��NH3���ӵĿռ乹����_________��N2H4�����е�ԭ�ӹ�����ӻ�������_______��
�����������ᷴӦ����N2H6SO4��N2H6SO4�����������������ͬ����N2H6SO4�ľ����ڲ�����__________(����)
a. ���Ӽ� b. ���ۼ� c. ��λ�� d. ���»���
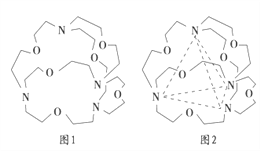
��ͼ1��ʾij�ֺ����л�������Ľṹ���������4����ԭ�ӷֱ�λ�����������4�����㣨��ͼ2���������ڴ��ڿ�ǻ����Ƕ��ij���ӻ���Ӳ��γ�4���������ʶ�����з��ӻ������У��ܱ����л�������ʶ�����________�����ţ���
a. CF4 b. NH4+ c.CH4 d. H2O
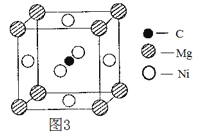
��3��������֣�ֻ��C��Mg��Ni����Ԫ�ص�ij�־�����г����ԡ��þ����һ��������ͼ3��ʾ����þ���Ļ�ѧʽΪ ___________��
���𰸡� 1s22s22p63s23p63d104s24p1 N��O��C HF ������ sp3 d b MgCNi3
��������(1)31GaΪ31��Ԫ�أ���̬ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s24p1��C��N��O����ͬһ����Ԫ����ԭ���������μ�С��ͬһ����Ԫ�صĵ�һ����������ԭ���������������������A��Ĵ��ڵ���A��ģ��������һ�����ܴ�С˳����N��O��C���� OH- ��Ϊ�ȵ�����ķ�����HF��HCl�ȣ��ʴ�Ϊ��1s22s22p63s23p63d104s24p1��N��O��C��HF��
(2)��NH3�����е�ԭ�Ӻ���3�����ۼ���һ���µ��Ӷԣ����Կռ乹���������ͣ�N2H4�����е�ԭ�ӵļ۲���Ӷ�=3+1=4������һ���µ��Ӷԣ�Nԭ�ӹ�����ӻ�������sp3���ʴ�Ϊ�������ͣ�sp3��
��3N2H6SO4��(NH4)2SO4�������Ӿ��壬N2H6 2+��SO42-֮��������Ӽ���N2H62+��N��H֮���γ�6�����ۼ�(����2����λ��)��N��N֮���γɹ��ۼ���SO42-��S��O֮���γɹ��ۼ����������»�������ѡ��d��
����������γ��������ɼ�Ԫ��(N��O��F��H)��֪��������Ƕ��ij���ֱ���4��Nԭ���γ�4��������ɳɼ�Ԫ�ؼ���Ŀ��֪ΪNH4+���ʴ�Ϊ��b��
(3)���ݾ���ͼ����֪��Cԭ��Ϊ���ģ�ԭ����Ϊ1��Mgԭ��λ�ڶ����ϣ�ԭ����Ϊ8��![]() =1��Niԭ��λ�������ϣ�ԭ����Ϊ6��
=1��Niԭ��λ�������ϣ�ԭ����Ϊ6��![]() =3�����Ըþ���Ļ�ѧʽΪMgCNi3���ʴ�Ϊ��MgCNi3��
=3�����Ըþ���Ļ�ѧʽΪMgCNi3���ʴ�Ϊ��MgCNi3��