��Ŀ����
��Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ�����仯����㷺Ӧ�������Ų��ϡ��������ϡ���ȼ�ϲ��ϡ����ϲ��ϵȸ��²�������
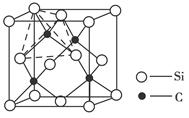
��1�����������ڳ��³�ѹ��Ϊ���д̱Ƕ����ǿ�̼��Ե���ɫ�ж���ʴ�����壬����ӵ����幹��Ϊ________��Bԭ�ӵ��ӻ�����Ϊ________��
��2��������һ���ܵ��߶ȹ�ע����ĥͿ�ϣ��������������ı��汣���㡣ͼ��a����������ľ���ʾ��ͼ��������Ļ�ѧʽΪ________���þ���ľ���������________��
��3�������ᣨH3BO3����һ��Ƭ��״�ṹ��ɫ���壬���ڵ�H3BO3���Ӽ�ͨ���������[��ͼ��b��]��
�����������B�������________�����ӣ�1 mol H3BO3�ľ�������________mol�����
����������ˮ�����������һˮ������B��OH��3��H2O����������������[B��OH��4]����H�����ӡ�������Ϊ________Ԫ�ᣬ[B��OH��4]�����еĻ�ѧ������Ϊ________��

��1�����������ڳ��³�ѹ��Ϊ���д̱Ƕ����ǿ�̼��Ե���ɫ�ж���ʴ�����壬����ӵ����幹��Ϊ________��Bԭ�ӵ��ӻ�����Ϊ________��
��2��������һ���ܵ��߶ȹ�ע����ĥͿ�ϣ��������������ı��汣���㡣ͼ��a����������ľ���ʾ��ͼ��������Ļ�ѧʽΪ________���þ���ľ���������________��
��3�������ᣨH3BO3����һ��Ƭ��״�ṹ��ɫ���壬���ڵ�H3BO3���Ӽ�ͨ���������[��ͼ��b��]��
�����������B�������________�����ӣ�1 mol H3BO3�ľ�������________mol�����
����������ˮ�����������һˮ������B��OH��3��H2O����������������[B��OH��4]����H�����ӡ�������Ϊ________Ԫ�ᣬ[B��OH��4]�����еĻ�ѧ������Ϊ________��

��1��ƽ�������Ρ�sp2
��2��BP��ԭ�Ӿ���
��3����6��3����һ�����ۼ�����λ��
��2��BP��ԭ�Ӿ���
��3����6��3����һ�����ۼ�����λ��
��1��BF3�����У�Bԭ���γ���3���Ҽ��������µ��Ӷԣ����ӻ������Ϊ3���ӻ���ʽΪsp2�ӻ���BF3�������幹��Ϊƽ�������Ρ���2��һ�������к���Bԭ�ӵĸ���Ϊ8��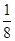 ��6��
��6��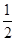 ��4����Pԭ�Ӹ���Ϊ4�����ʻ�ѧʽΪBP����������ֻ�����ۼ�����ĥ��Ϊԭ�Ӿ��塣��3������ͼ��֪��Bԭ���γ����������۵���������������Ϊ6��1 mol H3BO3�ľ����к���3 mol Hԭ�ӣ�ÿ��Hԭ�Ӷ������ڵ�Oԭ���γ���������������Ϊ3 mol����1 mol B��OH��3��H2O�ɵ����1 mol H����������ΪһԪ�[B��OH��4]��������Bԭ����һ���յ�2p�������OH�����йµ��Ӷԣ�����֮������γ���λ����
��4����Pԭ�Ӹ���Ϊ4�����ʻ�ѧʽΪBP����������ֻ�����ۼ�����ĥ��Ϊԭ�Ӿ��塣��3������ͼ��֪��Bԭ���γ����������۵���������������Ϊ6��1 mol H3BO3�ľ����к���3 mol Hԭ�ӣ�ÿ��Hԭ�Ӷ������ڵ�Oԭ���γ���������������Ϊ3 mol����1 mol B��OH��3��H2O�ɵ����1 mol H����������ΪһԪ�[B��OH��4]��������Bԭ����һ���յ�2p�������OH�����йµ��Ӷԣ�����֮������γ���λ����
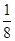 ��6��
��6��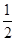 ��4����Pԭ�Ӹ���Ϊ4�����ʻ�ѧʽΪBP����������ֻ�����ۼ�����ĥ��Ϊԭ�Ӿ��塣��3������ͼ��֪��Bԭ���γ����������۵���������������Ϊ6��1 mol H3BO3�ľ����к���3 mol Hԭ�ӣ�ÿ��Hԭ�Ӷ������ڵ�Oԭ���γ���������������Ϊ3 mol����1 mol B��OH��3��H2O�ɵ����1 mol H����������ΪһԪ�[B��OH��4]��������Bԭ����һ���յ�2p�������OH�����йµ��Ӷԣ�����֮������γ���λ����
��4����Pԭ�Ӹ���Ϊ4�����ʻ�ѧʽΪBP����������ֻ�����ۼ�����ĥ��Ϊԭ�Ӿ��塣��3������ͼ��֪��Bԭ���γ����������۵���������������Ϊ6��1 mol H3BO3�ľ����к���3 mol Hԭ�ӣ�ÿ��Hԭ�Ӷ������ڵ�Oԭ���γ���������������Ϊ3 mol����1 mol B��OH��3��H2O�ɵ����1 mol H����������ΪһԪ�[B��OH��4]��������Bԭ����һ���յ�2p�������OH�����йµ��Ӷԣ�����֮������γ���λ����
��ϰ��ϵ�д�
�����Ŀ



 ����HCHO,����̼ԭ�Ӳ�ȡsp2�ӻ��ķ����� (���������),HCHO���ӵ�����ṹΪ ��;
����HCHO,����̼ԭ�Ӳ�ȡsp2�ӻ��ķ����� (���������),HCHO���ӵ�����ṹΪ ��;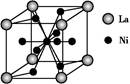

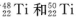 ����ԭ�ӣ����ǻ���Ϊ____��Ԫ����Ԫ�����ڱ��е�λ���ǵ�_____���ڣ���____�壻��̬ԭ�ӵĵ����Ų�ʽΪ_____���������Ų�TiԪ����Ԫ�����ڱ�����������_____��Ԫ�ء�
����ԭ�ӣ����ǻ���Ϊ____��Ԫ����Ԫ�����ڱ��е�λ���ǵ�_____���ڣ���____�壻��̬ԭ�ӵĵ����Ų�ʽΪ_____���������Ų�TiԪ����Ԫ�����ڱ�����������_____��Ԫ�ء�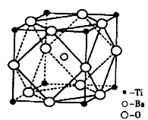
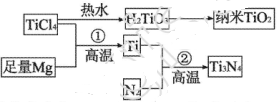 ��
��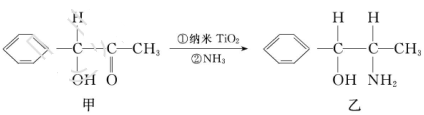
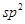 �ӻ���̼ԭ�Ӹ���Ϊ_____�����������в�ȡ
�ӻ���̼ԭ�Ӹ���Ϊ_____�����������в�ȡ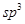 �ӻ���ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ_____��
�ӻ���ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ_____��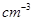 (NAΪ�����ӵ���������ֵ��ֻ����ʽ)��
(NAΪ�����ӵ���������ֵ��ֻ����ʽ)��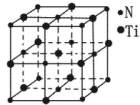
 )������˵����ȷ����________(�����)��
)������˵����ȷ����________(�����)��

