��Ŀ����
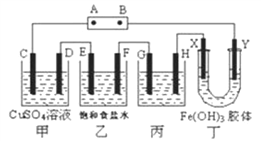
����Ŀ������ʽ�о���ѧϰ������ѧ������˼ά�����÷�����֣��һ��ѧϰС�齫����װ����ͼ���ӣ�D��F��X��Y ���Dz��缫��C��E �����缫������Դ��ͨ�������е����̪��Һ����F�������Ժ�ɫ���Իش�����������
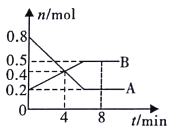
��1����ԴB ����������_______________��
��2����װ���е�ⷴӦ���ܻ�ѧ����ʽ����______________________��
��3����������Һ��������ͬһʱ��C��D �缫�ϲμӷ�Ӧ�ĵ��ʻ����ɵĵ��ʵ����ʵ���֮����________________��
��4�����ñ�װ�ý���ͭ(����������п������) ������G ������Ӧ����___________(������ͭ��������ͭ��)�����Һ��ԭ����ʵ����ʵ���Ũ�Ƚ�________(�������������С������������)��
��5��װ�ö��е�������__________________________��
��6����׳�����Һ������ڵ��ǰ����500ml�����ҳ���������������Ϊ4.48L (��״��) ʱ���׳������������ʵ����ʵ���Ũ��Ϊ__________mol��L-1��
���𰸡� ���� CuSO4+Fe![]() Cu+FeSO4 1��1 ��ͭ ��С Y���������ɫ���� 0.4
Cu+FeSO4 1��1 ��ͭ ��С Y���������ɫ���� 0.4
��������(1)�����е����̪��Һ����F�������Ժ�ɫ��˵���ü��������ӷŵ磬���Ըõ缫������������E�缫��������D�缫��������C�缫��������G�缫��������H�缫��������X�缫��������Y��������A�ǵ�Դ��������B��ԭ��صĸ������ʴ�Ϊ��������
(2)D��F��X��Y���Dz��缫��C��E�����缫���׳���CΪ����������DΪ���缫���������Һ������ͭ��Һ���������ͭ��Һʱ����������ʧ���������������ӣ�������ͭ���ӷŵ磬���Ե�ⷴӦΪ��Fe+CuSO4![]() Cu+FeSO4���ʴ�Ϊ��Fe+CuSO4
Cu+FeSO4���ʴ�Ϊ��Fe+CuSO4![]() Cu+FeSO4��
Cu+FeSO4��
(3)�׳���CΪ����������DΪ���缫���������Һ������ͭ��Һ���������ͭ��Һʱ����������ʧ���������������ӣ�Fe-2e-=Fe2+��������ͭ���ӷŵ磬�缫��ӦΪCu2++2e-=Cu�����ݵ����غ��֪��ͬһʱ��C��D�缫�ϲμӷ�Ӧ�ĵ��ʻ����ɵĵ��ʵ����ʵ���֮����1��1���ʴ�Ϊ��1��1��
(4)��⾫��ͭʱ����ͭ����������ͭ������������ͭ���ӵ���������ʼ��ɣ������жϱ�װ����GΪ���ص�������H�缫��������G�缫Ϊ��ͭ��H�缫Ϊ��ͭ�����ݵ����غ�����������ܽ������ͭ������п���������ʽ���Ҳʧ���ӣ����Ե������Һ��ͭ����Ũ�ȼ�С�����Һ��ԭ����ʵ����ʵ���Ũ�ȼ�С���ʴ�Ϊ����ͭ����С��
(5)�������Ե��������ԭ�����������������к��еĴ�����ɵ����ӻ���������Y���ƶ�������Y���������ɫ�������ʴ�Ϊ��Y���������ɫ���
(6)�ҳ���EΪ������Fe-2e-=Fe2+��FΪ�������缫��Ӧ2H++2e-=H2�������ҳ���������������Ϊ4.48L(��״��)���������������ʵ���=0.2mol�������ҳص���ת��Ϊ0.4mol���׳�����������ʧ���������������ӣ�Fe-2e-=Fe2+����������������Ũ��=![]() =0.4mol/L���ʴ�Ϊ��0.4��
=0.4mol/L���ʴ�Ϊ��0.4��