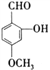
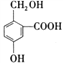
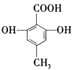
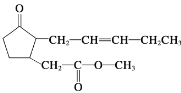
��Ŀ����
����Ŀ��NA Ϊ�����ӵ�������ֵ������˵����ȷ����( )
A.18gD2O �� 18gH2O �к��е���������Ϊ 10NA
B.12 g ʯīϩ(����ʯī)�к�����Ԫ���ĸ���Ϊ 0.5 NA
C.��״���£�5.6LCO2 ������ Na2O2 ��Ӧת�Ƶĵ�����Ϊ0.5 NA
D.ij�ܱ�����ʢ�� 1 mol N2 �� 3 mol H2����һ�������³�ַ�Ӧ��ת�Ƶ��ӵ���ĿΪ 6NA
���𰸡�B
��������
A��D2O��Ħ������Ϊ![]() ����18gD2O�����ʵ���Ϊ0.9mol�����е�������Ϊ9 NA��A����
����18gD2O�����ʵ���Ϊ0.9mol�����е�������Ϊ9 NA��A����
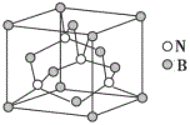
B������ʯī�Ľṹ����Ԫ����ɵģ�һ����Ԫ���к���![]() ��̼ԭ�ӣ�12gʯīϩ���ʵ���Ϊ1mol�����е���Ԫ������Ϊ0.5NA��B��ȷ��
��̼ԭ�ӣ�12gʯīϩ���ʵ���Ϊ1mol�����е���Ԫ������Ϊ0.5NA��B��ȷ��
C����״���£�5.6LCO2�����ʵ���Ϊ0.25mol����Na2O2��Ӧʱ��ת�Ƶ�����Ϊ0.25 NA��C����
D��N2��H2��ӦΪ���淴Ӧ������ת���ʲ�ȷ�������������ת�Ƶ��ӣ�D����
��ѡB��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ