��Ŀ����
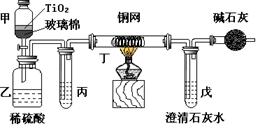
��֪�����������ķе�Ϊ213�棨�ڴ��¶�����ˮ���Ҵ��ͻ�������7��0%��17��0%��76��0%�ı�����Ϊ�����ݳ�������ش�����ʵ�����Ʊ��������������й����⣺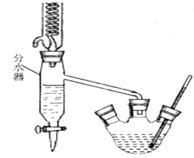
��1������������ƿ�м��뱽���ᡢŨ���ᡢ�������Ҵ�����ʯ�����������ƿ�м��뻷���飬װ�Ϸ�ˮ���Ļ�����ȴ�ܡ�ʵ����ʹ�÷�ˮ����Ŀ���ǣ��ӻ�ѧƽ��ԭ��������_______________��
��2���������Ȼ���������ˮ���²�Һ�岻�����ֹ࣬ͣ���ȣ��ų���ˮ����Һ�壬��ˮ����Һ���������Ҫ�ɷ���____________��
��3����Բ����ƿ�еIJ�Һ����ʢ����ˮ����ƿ�У���_____________��Һ�к��������Է�Һ���ֳ��ֲ�Ʒ��ˮ��������____________����ʵ��������ƣ����Ѳ���ֲ�Ʒ�ϲ����ô�ˮϴ�л������Σ����Ѳ���ˮ�����־����Ѳ���Ͽڵ���һ���������ƿ��
��4����������������С����ˮ�Ȼ��Ƹ������ҡ����ƿ�����Ѳ���������Ѳ������һ�������Բ����ƿ������____________����ʵ��������ƣ���������__________������������������
��1�����뷴Ӧ���������ɵ�ˮ���ٽ�������Ӧ����������У���2��ˮ���Ҵ��ͻ����飻
��3������̼������Һ ��ȡ��4������ ����
���������������1����������Ҵ���������ӦΪ���淴Ӧ��ˮΪ�����ʵ����ʹ�÷�ˮ����Ŀ���Ƿ��뷴Ӧ���������ɵ�ˮ���ٽ�������Ӧ����������У���2���������Ϣˮ���Ҵ��ͻ�������7��0%��17��0%��76��0%�ı�����Ϊ�����ݳ�����ˮ����Һ���������Ҫ�ɷ���ˮ���Ҵ��ͻ����飻��3��Ǩ�ƽ̲������������Ʊ�ʵ���ñ���̼������Һ���к�����ͱ����ᣬ�ܽ��Ҵ����ұ����������ڱ���̼������Һ�е��ܽ�Ȳ������ڱ������������������ѣ�����������ȡˮ���в���ı�������������4�������뱽�������������Ҷ��߷е㲻ͬ�����ѵķе�ϵͣ��ʲ�������ķ��������߷��룬�е�͵������ȱ��������������������������
���㣺�������ʵ��Ʊ�����������롣

 �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д� ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�ͨ������������������������ˮ�����ٷ�Һ�ŷŶԻ�������Ⱦ��ͬʱ����
K2Cr2O7��ʵ���ҶԺ�����Һ������Cr3+��Fe3+��K+��SO42����NO3��������Cr2O72���������������ù������£�
��֪����Cr(OH)3+OH��=CrO2��+2H2O��
��2CrO2��+3H2O2+2OH��=2CrO42��+4H2O��
��H2O2�����������¾��л�ԭ�ԣ��ܽ�+6��Cr��ԭΪ+3��Cr��
��1��ʵ��������KOHŨ��Ϊ6 mol��L��1������KOH��������250mL 6 mol��L��1 ��KOH��Һ�����ձ����������⣬�������õ��IJ��������� ��
��2�����ں�����Һ�к���������K2Cr2O7������ʱ���� ���沼��©�������˹���
��Ҫ��ʱ�۲�����ƿ��Һ��߶ȣ�����ﵽ֧�ܿ�λ��ʱӦ���еIJ���Ϊ ��
��3����Һ���ữǰ�����м��ȵ�Ŀ���� ����ԡ�����˺�Ӧ��������ˮϴ��K2Cr2O7����Ŀ���� ��
��4���±���������ʵ��ܽ�����ݣ�
| ���� | 0�� | 20�� | 40�� | 60�� | 80�� | 100�� |
| KCl | 28.0 | 34.2 | 40.1 | 45.8 | 51.3 | 56.3 |
| K2SO4 | 7.4 | 11.1 | 14.8 | 18.2 | 21.4 | 24.1 |
| K2Cr2O7 | 4.7 | 12.3 | 26.3 | 45.6 | 73.0 | 102.0 |
| KNO3 | 13.9 | 31.6 | 61.3 | 106 | 167 | 246.0 |
�����ܽ�����ݣ�����������������Ϊ�� ���� ��
��5����ȡ��Ʒ�ظ��������2.000g���250mL��Һ��ȡ��25.00mL����ƿ�У�����10mL 2 mol��L��1H2SO4�������⻯�ƣ����Ļ�ԭ����ΪCr3+�������ڰ���5min��Ȼ�����100mLˮ������3mL����ָʾ������0.1200 mol��L��1Na2S2O3����Һ�ζ���I2+2S2O32��=2I��+S4O62������
����ʵ���й���ȥNa2S2O3����Һ30.00mL�����ò�Ʒ�����ظ���صĴ���Ϊ ���������������������ʲ����뷴Ӧ����
�����ζ�����ʹ��ǰδ��Na2S2O3����Һ��ϴ����õ��ظ���صĴ��Ƚ��� ���ƫ�ߡ�����ƫ�͡����䡱����
1�������������������ϣ�Ҳ�ɺϳ��������ϡ�ʵ�����Ʊ�1�����������Ĺ������£� 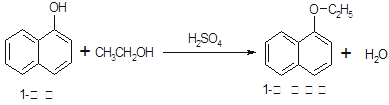
��֪����������ʵ���������
| ���� | ��Է� ������ | ״̬ | �۵�(��) | �е�(��) | �ܽ�� | |
| ˮ | �Ҵ� | |||||
| 1������ | 144 | ��ɫ���ɫ���νᾧ���ĩ | 96�� | 278�� | ����ˮ | �������Ҵ� |
| 1���������� | 172 | ��ɫҺ�� | 5��5�� | 267�� | ������ˮ | �������Ҵ� |
| �Ҵ� | 46 | ��ɫҺ�� | -114��1�� | 78��5�� | ����Ȼ��� | |
��1�����ӵ������뱽�����ƣ������ŵı�����ζ�������ڿ������ױ�����Ϊ�ۺ�ɫ��
��ش��������⣺
��1����72g1����������100mL��ˮ�Ҵ��У�����5mLŨ�����ϡ������Һ������ͼ��ʾ�������м��ȳ�ַ�Ӧ��ʵ����ʹ�ù����Ҵ���ԭ���� ��

��2����Ӧ����������ƿ�е�Һ�嵹����ˮ�У��������õ��л��㡣Ϊ�ᴿ�����������IJ�������������ˮϴ����Һ������10%��NaOH��Һ��ϴ����Һ��������ˮ�Ȼ��Ƹ��ﲢ���ˡ���ȷ��˳���� (�����)��
A���ۢڢܢ� B���٢ڢۢ� C���ڢ٢ۢ�
��3��ʵ����1�����������IJ����뷴Ӧʱ�䡢�¶ȵı仯��ͼ��ʾ��ʱ���ӳ����¶����ߣ�1�����������IJ����½���ԭ������� ��

��4���ᴿ�IJ�Ʒ���ⶨΪ43g����ʵ����1�����������IJ���Ϊ ��
��ͼ����ѧ��ѧ�г����ڻ����ķ�����ᴿ��װ�ã������װ�ûش����⣺
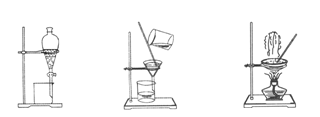
ͼ1 ͼ2 ͼ3 ͼ4
��1��װ��ͼ1��A��������________��B�������� ��A ��һ��Ҫ�������Ƭ���������� ��װ��ͼ4��ʢ��Һ������������ ��
��2��Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
1)���պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ��������__________��������������ѡ�������������ñ����ĸ��д�ڿհ״�����
| A���ձ� | B������ | C�������� | D�������� E���ƾ��� F�������� |
ѡ������װ��ͼ ����ͼ����ţ��������Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ⣬ͬʱ���ձ����ò����ʵ�����������________��ѡ������װ��ͼ ����ͼ����ţ�






 ������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00mL����ش��������⣺
������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00mL����ش��������⣺