��Ŀ����
7��ʵ���ҿ����ø�����غ�Ũ���ᷴӦ��ȡ��������Ӧ�Ļ�ѧ����ʽ���£�2KMnO4+16HCl��Ũ���T2KCl+2MnCl2+5Cl2��+8H2O��1���õ����ŷ��������ת�Ƶķ������Ŀ
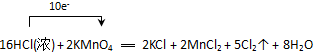 ��
����2���÷�Ӧ�е��������뻹ԭ�����ʵ���֮����1��5��
��3��KMnO4�������Ա�Cl2��������ǿ��ѡ�ǿ������������
��4���練Ӧ��ת����2mol���ӣ��������Cl2�ڱ�״�������Ϊ22.4L��
��5��ijͬѧ����KMnO4��������100mL0.5mol��L-1����Һ���ش��������⣺
������KMnO4��Һʱ���õ���Ҫ������������ƽ��ҩ�ס��ձ�������������Ͳ����ͷ�ιܡ�100mL����ƿ��
��Ӧ��������ƽ��ȡKMnO4����7.9g��
�۲��淶��ʵ������ᵼ��ʵ���������������в�����ʵ������Ӱ��ƫС���ǣ�������ţ�CD
A����ˮ����ʱ���ӿ̶���
B������ƿ�ڱڸ���ˮ���δ���ﴦ��
C���ߵ�ҡ�Ⱥ��ְ�Һ����ڿ̶����ּ�ˮ����
D�����ܽ������������Һ�彦���ձ��⣮
���� �ɷ���ʽ��֪����Ӧ��MnԪ�ػ��ϼ���+7�۽��͵�+2�ۣ���������KMnO4Ϊ��������ClԪ�ػ��ϼ���-1�����ߵ�0�ۣ���������HClΪ��ԭ������ϻ��ϼ۵ı仯�Լ���Ӧ�ķ���ʽ�ɼ���ת�Ƶ��ӵ���Ŀ���Դ˽��1������4����
��5��KMnO4��������100mL 0.5mol��L-1����Һ����Ҫ100mL����ƿ����ͷ�ιܣ������n=cV��m=nM��c=$\frac{n}{V}$���
��� �⣺��1����Ӧ��KMnO4Ϊ��������MnԪ�صĻ��ϼ���+7�۽��͵�+2�ۣ��õ�5�����ӣ�ClԪ�ػ��ϼ����ߣ�������������2molKMnO4�μӷ�Ӧ��ת�Ƶ���10mol�������ŷ��������ת�Ƶķ������ĿΪ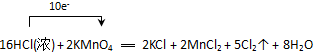 ��
��
�ʴ�Ϊ��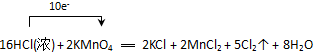 ��
��
��2���ɷ�Ӧ��֪��2molKMnO4������������10molHCl����ԭ������ʧ������ͬ����÷�Ӧ�е��������뻹ԭ�����ʵ���֮����1��5���ʴ�Ϊ��1��5��
��3��������ΪKMnO4����������Ϊ���������������������Դ�����������������Կ�֪��KMnO4�������Ա�Cl2��������ǿ���ʴ�Ϊ��ǿ��
��4���練Ӧ��ת����2mol���ӣ��������Cl2�ڱ�״�������Ϊ$\frac{2mol}{��1-0����2}$��22.4L/mol=22.4L���ʴ�Ϊ��22.4��
��5����KMnO4��������100mL 0.5mol��L-1����Һ����Ҫ100mL����ƿ��������Ҫ��ͷ�ιܣ��ʴ�Ϊ����ͷ�ιܣ�100mL����ƿ��
����Ҫ���������Ϊ0.1L��0.5mol/L��158g/mol=7.9g���ʴ�Ϊ��7.9��
��A����ˮ����ʱ���ӿ̶��ߣ���Һ�����ƫС����Ũ��ƫ��A��ѡ��
B������ƿ�ڱڸ���ˮ���δ���ﴦ�����������趨�ݼ�ˮ����ʵ����Ӱ�죬��B��ѡ��
C���ߵ�ҡ�Ⱥ��ְ�Һ����ڿ̶����ּ�ˮ���ϣ���Һ���ƫ����Ũ��ƫС����Cѡ��
D�����ܽ������������Һ�彦���ձ��⣬���ʼ��٣���������ҺŨ��ƫС����Dѡ��
�ʴ�Ϊ��CD��
���� ���⿼��������ԭ��Ӧ�������Һ�����ƣ�Ϊ��Ƶ���㣬���շ�Ӧ��Ԫ�صĻ��ϼ۱仯����Һ���Ʋ���Ϊ���Ĺؼ������ػ������ת�Ƶ��Ӽ�ʵ����������Ŀ��飬��Ŀ�ѶȲ���

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�| A�� | 0.5 mol•L-1 | B�� | l mol•L-1 | C�� | 2 mol•L-1 | D�� | 3 mol•L-1 |
| A�� | K2CO3��Һ��c��K+����c��CO32-��֮�� | |
| B�� | 0.2mol/L��CH3COOH��Һ��0.1mol/L��������c��H+��֮�� | |
| C�� | pH=7�İ�ˮ�루NH4��2SO4�Ļ����Һ�У�c��NH4+����c��SO42-��֮�� | |
| D�� | pH=12��KOH��Һ��pH=12��Ba��OH��2��Һ�����ʵ����ʵ���Ũ��֮�� |
| A�� | ������Ϊ17��������Ϊ20����ԭ�ӣ�${\;}_{17}^{20}$Cl | |
| B�� | �����ӣ�Cl-���Ľṹʾ��ͼ��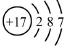 | |
| C�� | �ȷ��ӵĵ���ʽ��${\;}_{•}^{•}$$\underset{\stackrel{••}{Cl}}{••}$${\;}_{•}^{•}$$\underset{\stackrel{••}{Cl}}{••}$${\;}_{•}^{•}$ | |
| D�� | ����ϩ���ӵĽṹ��ʽ��H3C-CH2Cl |
| ѡ�� | Ŀ�� | ���� |
| A | ����100 mL 1.0 mol/L CuSO4��Һ | ��25 g CuSO4•5H20����100 mL����ˮ�� |
| B | ��ȥKNO3������NaCl | ��������Ƴ��ȵ�Ũ��Һ����ȴ�ᾧ�����ˡ�ϴ�� |
| C | ����ij��Һ���Ƿ������ | ����Һ���ȼ��������Ȼ��� |
| D | ȷ��NaCl��Һ���Ƿ����Na2CO3 | ȡ������Һ�μ�CaCl2��Һ���۲��Ƿ���ְ�ɫ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ԭ���ƴ���̼���� | |
| B�� | ���ƴ�������ܴ�Ӧ�� | |
| C�� | ���ƴ��ij���������� | |
| D�� | ���ƴ������������棬���������������ɿ������Ȼ��� |
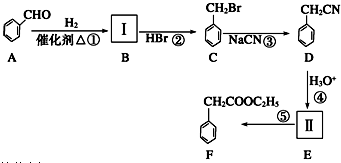
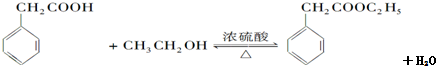 ��Ҫ��д����Ӧ��������
��Ҫ��д����Ӧ�������� ��Ҫ��д����Ӧ��������
��Ҫ��д����Ӧ�������� ��
��