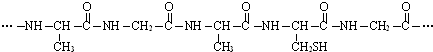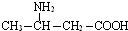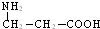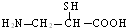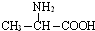��Ŀ����
19����֪����ѧ���ݣ�Na��s����Na+��g��+Cl-��g����H1=553.79kJ•mol-1
Na+��g��+e-��Na��g����H2=-487.55kJ•mol-1
Cl-��g��-e-��Cl��g��+e-��H3=-348.29kJ•mol-1
�Ը����Ȼ�ѧ���㣬˵��NaCl��s���ڸ���������������Na��g����Cl��g��ԭ�ӻ�������Na+��g����Cl-��g�����ӣ�����۶��к�ʵ����ݣ�
���� NaCl��s����Na+��g��+Cl-��g����H1=553.79kJ•mol-1�٣�
Na+��g��+e-��Na��g����H2=-487.55kJ•mol-1�ڣ�
Cl-��g��-e-��Cl��g��+e-��H3=-348.29kJ•mol-1�ۣ�
���ݸ�˹���ɣ���+��+�۵õ�NaCl��s����Na��g��+Cl��g����H1����ϡ�H-T•��S��0��Ӧ�Է������������жϣ�
��� �⣺NaCl��s����Na+��g��+Cl-��g����H1=553.79kJ•mol-1�٣�
Na+��g��+e-��Na��g����H2=-487.55kJ•mol-1�ڣ�
Cl-��g��-e-��Cl��g��+e-��H3=-348.29kJ•mol-1�ۣ�
���ݸ�˹���ɣ���+��+�۵õ�NaCl��s����Na��g��+Cl��g����H1=553.79kJ•mol-1+��-487.55kJ•mol-1��+��-348.29kJ•mol-1��=-282.05kJ•mol-1��
��֪����H-T•��S��0��Ӧ�Է����У�
�÷�Ӧ������Ϊ�������ʵ�������ķ���������S��0�����ԡ�H-T•��S=-282.05kJ•mol-1-T•��S��0����Ӧ���Է����У����NaCl��s���ڸ���������������Na��g����Cl��g��ԭ�ӣ�
��NaCl��s���ڸ�������������Na��g����Cl��g��ԭ�ӣ��÷�Ӧ��H-T•��S��0���ڸ��������Է����У�
���� ���⿼���˸�˹���ɵ�Ӧ�á���Ӧ�Է��Ե��жϣ���Ŀ�Ѷ��еȣ�ע����ݷ�Ӧ����������ʵ����仯���ж��ر䣮

| A�� | CH4 | B�� | CO2 | C�� | H2S | D�� | N2 |
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | C2H5OHŨ����170�湲�ȣ� �Ƶ�����ͨ������KmnO4��Һ | �����Ƶ������Ƿ�Ϊ��ϩ |
| B | ����������FeBr2��FeCl2��Һ�� ����������ˮ���ټ�CCl4��ȡ��Һ | ��ȥFeCl2��Һ�е�FeBr2 |
| C | �ڵ�����Һ�еμ���������ʳ�����Ƶ���Һ | ������ٵ��� |
| D | ������Һ�е����������ΪC2H4O2��Һ̬ �л��ˮ���ȣ����������� | ����ȷ�����л���һ���Ǽ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �����¶� | B�� | ������Ƭ������ | ||
| C�� | �����۴�����Ƭ | D�� | ��98%��Ũ�������ϡ���� |
| A�� | �����������£�CH3CO18OC2H5ˮ��IJ�����CH3CO18OH��C2H5OH | |
| B�� | �øʰ��ᣨH2NCH2COOH���ͱ����ᣨCH3CHNH2COOH��������ϣ������γ�4�ֶ��� | |
| C�� | ����ȩ�л��б����ᣬ������ʯ�ң��ټ������� | |
| D�� | ����ʯ�뱥��ʳ��ˮ��Ӧ���ɵ�����ͨ��������Ȼ�̼��Һ�У�����Һ��ɫ��֤������Ȳ���� |
| A�� | �ʵ������¶� | B�� | ��п����Ϊп�� | ||
| C�� | ���������������ˮ�ʵ�ϡ�� | D�� | Ѱ��һ�ֺ��ʵĴ������������� |
| A�� | �Ʒ��ڿ������ױ���������Ϊ�������ƣ����Ӧ������ˮ���� | |
| B�� | ����ǿ��ԭ�������ܴ�ˮ��Һ���û��������˳������ƺ���Ľ��� | |
| C�� | ����������������Ƴ��Ƶƣ������ں����������� | |
| D�� | �������Ż�ʱ������ˮ����� |
 ��
��