��Ŀ����
����Ŀ����NAΪ�����ӵ���������ֵ������˵���в���ȷ����
A. ��״���£�22.4L C2H4��22.4L������C-H������Ŀ��Ϊ6NA
B. 44g��N2O��CO2��ɵĻ��������������ԭ������Ϊ3NA
C. 2.8g��CO��C2H4��ɵĻ�������������ķ�������Ϊ0.1NA
D. 1L 0.1mol��L-1CH3COOH��Һ�У�CH3COOH��CH3COO-����Ŀ֮��Ϊ0.1NA
���𰸡�A
��������A����ڱ�״���³�Һ̬��������22.4L/mol���㱽�����ʵ���������B�N2O��CO2��Ħ����������44g/mol����44g��N2O��CO2��ɵĻ�������к�����������ʵ���Ϊ![]() =1mol��N2O��CO2������ԭ�ӷ��ӣ���ԭ�����ʵ���Ϊ3mol����ȷ��C�CO��C2H4��Ħ����������28g/mol����2.8g��CO��C2H4��ɵĻ�������к�����������ʵ���Ϊ
=1mol��N2O��CO2������ԭ�ӷ��ӣ���ԭ�����ʵ���Ϊ3mol����ȷ��C�CO��C2H4��Ħ����������28g/mol����2.8g��CO��C2H4��ɵĻ�������к�����������ʵ���Ϊ![]() =0.1mol����ȷ��D�CH3COOH�������ᣬ����Һ�д��ڵ���ƽ�⣺CH3COOH
=0.1mol����ȷ��D�CH3COOH�������ᣬ����Һ�д��ڵ���ƽ�⣺CH3COOH![]() CH3COO-+H+�����������غ�n��CH3COOH��+n��CH3COO-��=0.1mol/L
CH3COO-+H+�����������غ�n��CH3COOH��+n��CH3COO-��=0.1mol/L![]() 1L=0.1mol����ȷ����ѡA��
1L=0.1mol����ȷ����ѡA��

��ϰ��ϵ�д�
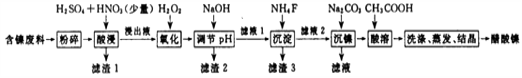
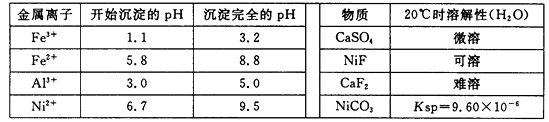
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ