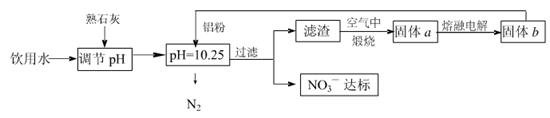
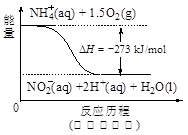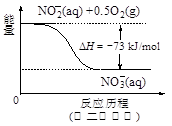��Ŀ����
ij��ɫ����Һ�п��ܺ���Ag+��Fe3+��K+��Ba2+��NH4+�������ӡ�ijͬѧ��������ʵ�飺
I�����������ϡ���ᣬ�а�ɫ�������ɡ�
II�����ˣ�ȡ������Һ�������м��������ϡ���ᣬ���а�ɫ�������ɡ�
III����ȡ��������II�е���Һ������NaOH��Һ����Һ�ʼ��ԣ����ȣ��ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����塣
��1������Һ��һ�����е�������__________��һ�������е�������___________��
��2������III�в�����������ӷ���ʽΪ__________________��
��1��Ag+��Ba2+��NH4+ Fe3+��2��NH4++OH�� NH3��+H2O
NH3��+H2O
���������������������֪����ҺΪ��ɫ����Fe3+ ��ˮ��Һ�����ػ�ɫ����һ��û�� Fe3+�����ݢ�֪�����������ϡ���ᣬ�а�ɫ�������ɣ���Һ��һ���� Ag+�����ݢ�֪�����������ϡ���ᣬ���а�ɫ�������ɣ���Һ�����ԣ���Һ��һ���� Ba2+�����ݢ�֪������NaOH��Һ����Һ�ʼ��ԣ����ȣ��ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬��Һ��һ���� NH4+��������������֪����1������Һ��һ�����е������� Ag+��Ba2+��NH4+�� һ�������е�������Fe3+���� 3����κ��������Ʒ�Ӧ���ӷ���ʽΪNH4++OH�� NH3��+H2O ��
NH3��+H2O ��
���㣺�������Ӽ���������ƶϡ�

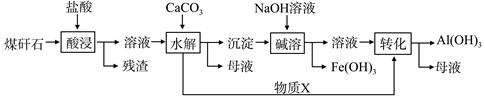
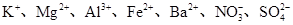 ��
��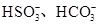 ��I-��Cl-��ȡ����Һ��������ʵ�飺
��I-��Cl-��ȡ����Һ��������ʵ�飺