��Ŀ����
ij��ú��ʯ��Ԥ������SiO2��63%����Al2O3��25%����Fe2O3��5%����������þ�Ļ�����ȣ�һ���ۺ����ù���������£�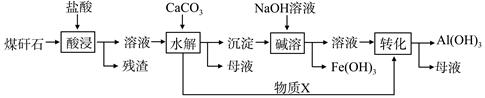
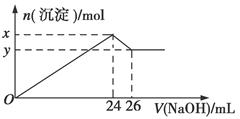
��1�����������������Ҫ��Ӧ�����ӷ���ʽΪ_____________��_________________��
��2���������ʱ�������ʵ�Ӱ�����ؿ�����_____________��___________����д��������
��3������X�Ļ�ѧʽΪ___________�������ܡ�ʱ��Ӧ�����ӷ���ʽΪ____________��
��4����֪Fe3+��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ2.1��3.2��Al3+��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ4.1��5.4��Ϊ�˻�ò�ƷAl��OH�� 3����ú��ʯ�������ȡҺ��ʼ����ֻ��CaCO3һ���Լ�����������������____________________��
��5����ú��ʯΪԭ�ϻ����Կ���������Ʒ��������ú��ʯ�������ȡҺ������������AlCl3��Һ�в���ͨ��HCl���壬����������AlCl3��6H2O���壬��ϻ�ѧƽ���ƶ�ԭ���������������ԭ��_______________________��
��1��Al2O3+6H+==2Al3++3H2O �� Fe2O3+6H+==2Fe3++3H2O ��4�֣�ÿ��2�֣�
��2�������Ũ�ȡ���Ӧ�¶ȡ�ú��ʯ������С���Ƿ��ֽ��衢��Ӧʱ�䣨��д��������4�֣�ÿ��2�֣�
��3��CO2�� Al��OH��3 +OH��=AlO2��+2H2O ��4�֣�ÿ��2�֣�
��4������CaCO3����pH��3.2�����˳�ȥFe��OH�� 3���ټ���CaCO3����pH��5.4�����˵õ�Al��OH�� 3 ��2�֣�
��5��AlCl3������Һ�д����ܽ�ƽ�⣺AlCl3��6H2O��s�� Al3+��aq�� +3Cl����aq�� +6H2O��l����ͨ��HCl����ʹ��Һ��c��Cl��������ƽ������������ķ����ƶ��Ӷ�����AlCl3���塣 ��2�֣�
Al3+��aq�� +3Cl����aq�� +6H2O��l����ͨ��HCl����ʹ��Һ��c��Cl��������ƽ������������ķ����ƶ��Ӷ�����AlCl3���塣 ��2�֣�
���������������1������ú��ʯ��Ԥ�����ɷֿ�֪������HCl��Ҫ��Ӧ��ΪAl2O3��Fe2O3���䷴Ӧ������
����ʽΪAl2O3+6H+==2Al3++3H2O�� Fe2O3+6H+==2Fe3++3H2O ��
��2��Ӱ����������������Ũ�ȡ���Ӧ�¶ȡ��������ú��ʯ������С�����Ƿ��ֽ��衢��Ӧʱ��ȣ�
��3����������Һ�����ԣ�һ������ʣ
���HCl����һ������Al3++3H2O Al��OH��3+3H+��Fe3++3H2O
Al��OH��3+3H+��Fe3++3H2O Fe��OH��3+3H+��ˮ������ԣ�����CaCO3������H+��Ӧ����CO2����Al��OH��3�������������������Һ�ᣬ��������ǿ��������
Fe��OH��3+3H+��ˮ������ԣ�����CaCO3������H+��Ӧ����CO2����Al��OH��3�������������������Һ�ᣬ��������ǿ��������
�����ʱ����Ӧ�����ӷ���ʽΪAl��OH��3 +OH��=AlO2��+2H2O��
��4������Fe3+��Al3+������pH��֪��ʹFe3+����ʱ��Al3+���Ӳ��ܳ�������Ӧ��pH������3.2��Ϊ�˻�ò�ƷAl��OH�� 3������ҪAl3+������ȫ�������ʽ�pH������5.4���ڵڶ�������pH֮ǰ��Ӧ�ù��˵ķ�����Fe��OH�� 3��ȥ����5��AlCl3������Һ�д����ܽ�ƽ�⣬ͨ��HCl��������ˮ�����Cl����ʹ��Һ��c��Cl��������ƽ������������ķ����ƶ��Ӷ�����AlCl3���塣
���㣺���黯ѧ��������ͼ����Ҫ���������ӷ���ʽ��Ӱ�컯ѧ��Ӧ�����ء�Ԫ�ؼ��仯��������ʡ�������Һ��pHֵ���ӡ������ܽ�ƽ�⡣

 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д�ij��Һ���ܺ���Cl����SO42����CO32����NH4+��Fe3+��Fe2+��Al3+��Na+��ijͬѧΪ��ȷ����ɷ֣�ȡ������Һ����Ʋ����������ʵ�飺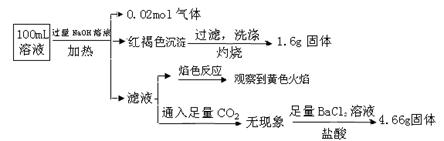
�ɴ˿�֪ԭ��Һ��
| A��ԭ��Һ��c��Fe3+��="0.2" mol��L-1 |
| B����Һ��������4�����Ӵ��ڣ�����Cl��һ�����ڣ���c��Cl������0.2 mol��L-1 |
| C��SO42����NH4+��Na+һ�����ڣ�CO32����Al3+һ�������� |
| D��Ҫȷ��ԭ��Һ���Ƿ���Fe2+,�����Ϊ��ȡ����ԭ��Һ���Թ���,����������ˮ���������ټ�KSCN��Һ����Һ��Ѫ��ɫ������Fe2+ |
ij����С���һЩ�������ʺͻ���������ʽ���̽����
��1���±�Ϊ�������Ȼ�ͭ��Һ��Ӧ��ʵ�鱨���һ���֣�
| ʵ�鲽�� | ʵ������ |
| ����ĥ������Ƭ������������һ��Ũ�ȵ�CuCl2��Һ�� | �������ݣ��������ɵĺ�ɫ���壬��Һ��Ϊ��ɫ |
| ��Ӧ������������Һ���� | |
| ��ɫ����������ˮϴ�Ӻ����ڳ�ʪ������ | һ��ʱ�������ɺ�ɫ��Ϊ��ɫ[������Ҫ�ɷ�ΪCu2��OH��2CO3] |
����Ӧ����д��ʵ���з�����Ӧ�Ļ�ѧ����ʽ��һ���������ӷ�Ӧ��ֻд���ӷ���ʽ��
�û���Ӧ_________________________________________________________��
���Ϸ�Ӧ_________________________________________________________��
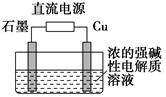
��2����ʯī���缫���������ʵ����������Һ�������������ݡ�������⣬��������������Һ�л����Թ۲쵽��������__________________________________________________��
���ʹ���������ӷ���ʽ��___________________________________________��
��3����ҵ�Ͽ����������̿���Ҫ�ɷ�ΪMnO2����Ӧ��ұ�������̡�
�����������̿����̵�ԭ���ǣ��û�ѧ����ʽ����ʾ��________________________��
��MnO2��H2O2�ֽⷴӦ������������������MnO2�����ữ��H2O2��Һ�У�MnO2�ܽ����Mn2�����÷�Ӧ�����ӷ���ʽ��_______________________��