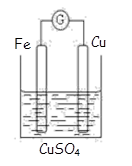
��Ŀ����
CuI��һ�ֲ�����ˮ�İ�ɫ���壬�����ɷ�Ӧ��2Cu2����4I��=2CuI����I2���õ�������ͭƬ��ʯī���缫�����KI��Һ��ȡCuI��Ϊȷ�Ϸ�Ӧ�����ͨ��ǰ����Һ���ּ����������ķ�̪��Һ�͵�����Һ�����һ��ʱ���õ���ɫ������ͬʱ��������Һ��죬��������Һ����������˵����ȷ���� (�� )
��ͭƬ��������ʯī������ ��ͭƬ��������ʯī������
���������������� ����������������
�ݰ�ɫ������������������ �ް�ɫ������������������
����������Һ������ԭ����2Cu��4I�D�D4e��=2CuI����I2 �������۱���
����������Һ������ԭ����4OH�D�D4e�D=2H2O��O2����O2��I������ΪI2���������۱���
A��ֻ�Тڢܢݢ� B��ֻ�Т٢ܢޢ� C��ֻ�Тڢۢݢ� D��ֻ�Т٢ۢޢ�
���𰸡�
A
����������

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
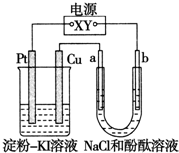 ��2011?�㽭��У����������CuI��һ�ֲ�����ˮ�İ�ɫ���壬�������ɷ�Ӧ��2Cu2++4I-�T2CuI��+I2���õ�����ͼ��ʾװ���У�a��b���Ƕ��Ե缫��ͨ��һ��ʱ����ڵ���-KI��Һ��������Χ����ɫ��������˵����ȷ���ǣ�������
��2011?�㽭��У����������CuI��һ�ֲ�����ˮ�İ�ɫ���壬�������ɷ�Ӧ��2Cu2++4I-�T2CuI��+I2���õ�����ͼ��ʾװ���У�a��b���Ƕ��Ե缫��ͨ��һ��ʱ����ڵ���-KI��Һ��������Χ����ɫ��������˵����ȷ���ǣ�������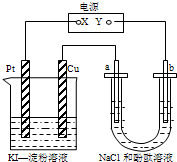 CuI��һ�ֲ�����ˮ�İ�ɫ���壬�������ɷ�Ӧ��2Cu2++4I-=2CuI��+I2���õ�����ͼ��ʾװ���У�a��b���Ƕ��Ե缫��ͨ��һ��ʱ�����KI-������Һ��������Χ����ɫ��������˵����ȷ���ǣ�������
CuI��һ�ֲ�����ˮ�İ�ɫ���壬�������ɷ�Ӧ��2Cu2++4I-=2CuI��+I2���õ�����ͼ��ʾװ���У�a��b���Ƕ��Ե缫��ͨ��һ��ʱ�����KI-������Һ��������Χ����ɫ��������˵����ȷ���ǣ�������