��Ŀ����
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�ء���֪H2(g)��CO(g)��CH3OH(l)��ȼ���ȡ�H�ֱ�Ϊ��285.8 kJ��mol��1����283.0 kJ��mol��1�ͣ�726.5 kJ��mol��1����ش��������⣺
��1����̫���ֽܷ�10molˮ���ĵ�������_____________kJ��
��2���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ________________________��
��3�����ݻ�Ϊ2 L���ܱ������У���CO2��H2�ϳɼ״�(CO2+3H2 CH3OH + H2O)
CH3OH + H2O)
�������������������£������¶ȶԷ�Ӧ��Ӱ�죬ʵ��������ͼ��ʾ��ע��T1��T2������300�棩������˵����ȷ����______������ţ�
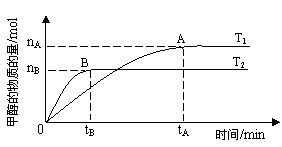
���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״���ƽ������Ϊv(CH3OH) ��nA/tA mol��L-1��min-1
�ڸ÷�Ӧ��T1ʱ��ƽ�ⳣ����T2ʱ��С
�۸÷�ӦΪ���ȷ�Ӧ
�ܴ���A��ķ�Ӧ��ϵ��T1�䵽T2���ﵽƽ��ʱn(H2)/n(CH3OH)����
��4����T1�¶�ʱ����1mol CO2��3mol H2����һ�ܱպ������У���ַ�Ӧ�ﵽƽ�����CO2ת����Ϊa,�������ڵ�ѹǿ����ʼѹǿ֮��Ϊ_________________________��
��1����̫���ֽܷ�10molˮ���ĵ�������_____________kJ��
��2���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ________________________��
��3�����ݻ�Ϊ2 L���ܱ������У���CO2��H2�ϳɼ״�(CO2+3H2
 CH3OH + H2O)
CH3OH + H2O)�������������������£������¶ȶԷ�Ӧ��Ӱ�죬ʵ��������ͼ��ʾ��ע��T1��T2������300�棩������˵����ȷ����______������ţ�

���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״���ƽ������Ϊv(CH3OH) ��nA/tA mol��L-1��min-1
�ڸ÷�Ӧ��T1ʱ��ƽ�ⳣ����T2ʱ��С
�۸÷�ӦΪ���ȷ�Ӧ
�ܴ���A��ķ�Ӧ��ϵ��T1�䵽T2���ﵽƽ��ʱn(H2)/n(CH3OH)����
��4����T1�¶�ʱ����1mol CO2��3mol H2����һ�ܱպ������У���ַ�Ӧ�ﵽƽ�����CO2ת����Ϊa,�������ڵ�ѹǿ����ʼѹǿ֮��Ϊ_________________________��
��1��2858 ��2��CH3OH(l)+O2(g)===CO(g)+2H2O(l) ��H=-443.5kJ/moL
��3���ۢ� ��4����4-2a����4
��3���ۢ� ��4����4-2a����4
��1��������ȼ�����ǣ�285.8 kJ��mol������1molˮ�ֽ���Ҫ����������285.8 kJ����ֽ�10molˮ����Ҫ2858 kJ��
��2�����������֪����CH3OH(l)+3/2O2(g)��CO2(g)+2H2O(l) ��H=-726.5kJ/moL����CO(g)+
1/2O2(g)��CO2(g) ��H=��283.0kJ/mol�����Ԣ٣��ڼ��õ�CH3OH(l)+O2(g)��CO(g)+2H2O(l)�����Է�Ӧ����-726.5kJ/moL��283.0kJ/mol��-443.5kJ/moL��
��3���ٲ���ȷ����Ϊƽ��ʱ�״���Ũ����nA/2 mol��L-1.T2ʱ���ȴﵽƽ��״̬�����¶���T2����T1���¶ȸ״��ĺ����ף�˵�������¶�ƽ�����淴Ӧ�����ƶ���������Ӧ�Ƿ��ȷ�Ӧ�����Ԣڲ���ȷ���ۢ���ȷ����ѡ�ۢܡ�
��4��CO2ת����Ϊa��������CO2ʱamol������ʱ3amol�����ɼ״���ˮ��������amol�����������ڵ�ѹǿ����ʼѹǿ֮��Ϊ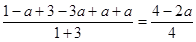 ��
��
��2�����������֪����CH3OH(l)+3/2O2(g)��CO2(g)+2H2O(l) ��H=-726.5kJ/moL����CO(g)+
1/2O2(g)��CO2(g) ��H=��283.0kJ/mol�����Ԣ٣��ڼ��õ�CH3OH(l)+O2(g)��CO(g)+2H2O(l)�����Է�Ӧ����-726.5kJ/moL��283.0kJ/mol��-443.5kJ/moL��
��3���ٲ���ȷ����Ϊƽ��ʱ�״���Ũ����nA/2 mol��L-1.T2ʱ���ȴﵽƽ��״̬�����¶���T2����T1���¶ȸ״��ĺ����ף�˵�������¶�ƽ�����淴Ӧ�����ƶ���������Ӧ�Ƿ��ȷ�Ӧ�����Ԣڲ���ȷ���ۢ���ȷ����ѡ�ۢܡ�
��4��CO2ת����Ϊa��������CO2ʱamol������ʱ3amol�����ɼ״���ˮ��������amol�����������ڵ�ѹǿ����ʼѹǿ֮��Ϊ
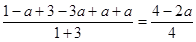 ��
��
��ϰ��ϵ�д�
 ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д�
�����Ŀ
 Cd(OH)2 + 2Ni(OH)2����֪Ni(OH)2��Cd(OH)2��
Cd(OH)2 + 2Ni(OH)2����֪Ni(OH)2��Cd(OH)2��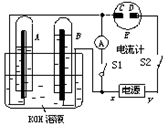
 2PbSO4��2H2O��������������ȷ����(����)
2PbSO4��2H2O��������������ȷ����(����) 2Na+xS������˵����ȷ����
2Na+xS������˵����ȷ���� Li2S2O4�������йظõ�ص�˵����ȷ���ǣ� ��
Li2S2O4�������йظõ�ص�˵����ȷ���ǣ� ��