��Ŀ����
��X��Y��Z��W����Ԫ�أ����ǵĵ���X��Y��Z�ڳ��³�ѹ�½�Ϊ���壬WΪ���塣
��X���ʿ���Z������ȼ������XZ�������Ϊ��ɫ��W������Y�����о���ȼ�ղ�����ɫ���棬����W2Y2��
��ÿ2molX2����1molY2��������2molX2Y��X2Y�ڳ�����ΪҺ�壻
�ۻ�����XZ��ˮ��Һ��ʹ��ɫʯ����ֽ��죻W��ˮ��Ӧ�����Һ��ʹ��ɫ��̪��Һ���ɫ��
��Z�������ڻ�����X2Y��������Һ����Ư�����ã���ش�
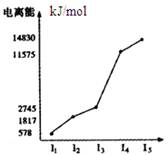
��1������Zԭ�ӵ�ԭ�ӽṹʾ��ͼ ��ʵ�����е�WӦ������____________�У����Լ����ƣ�
��2��д����ѧʽW2Y2___________ _
��3��д����W+������ͬ����������ķ��ӻ�ԭ�ӣ���д������3�ֵĻ�ѧʽ
�� �� ��
��4��Z������ˮ��Ӧ�����ӷ���ʽ��_________________________
��W������X2Y��ѧ��Ӧ����ʽ��__________________________
��X���ʿ���Z������ȼ������XZ�������Ϊ��ɫ��W������Y�����о���ȼ�ղ�����ɫ���棬����W2Y2��
��ÿ2molX2����1molY2��������2molX2Y��X2Y�ڳ�����ΪҺ�壻
�ۻ�����XZ��ˮ��Һ��ʹ��ɫʯ����ֽ��죻W��ˮ��Ӧ�����Һ��ʹ��ɫ��̪��Һ���ɫ��
��Z�������ڻ�����X2Y��������Һ����Ư�����ã���ش�
��1������Zԭ�ӵ�ԭ�ӽṹʾ��ͼ ��ʵ�����е�WӦ������____________�У����Լ����ƣ�
��2��д����ѧʽW2Y2___________ _
��3��д����W+������ͬ����������ķ��ӻ�ԭ�ӣ���д������3�ֵĻ�ѧʽ
�� �� ��
��4��Z������ˮ��Ӧ�����ӷ���ʽ��_________________________
��W������X2Y��ѧ��Ӧ����ʽ��__________________________
��1��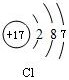 (2��) ú�� (2��) ��2��Na2O2 (2��)
(2��) ú�� (2��) ��2��Na2O2 (2��)
��3��CH4 HF NH3 H2O Ne (��ѡ3����ÿ��1�֣���3��)
��4��Cl2+H2O H++Cl-+HClO (2��) �� 2Na+2H2O=2NaOH+H2��(2��)
H++Cl-+HClO (2��) �� 2Na+2H2O=2NaOH+H2��(2��)
 (2��) ú�� (2��) ��2��Na2O2 (2��)
(2��) ú�� (2��) ��2��Na2O2 (2��) ��3��CH4 HF NH3 H2O Ne (��ѡ3����ÿ��1�֣���3��)
��4��Cl2+H2O
 H++Cl-+HClO (2��) �� 2Na+2H2O=2NaOH+H2��(2��)
H++Cl-+HClO (2��) �� 2Na+2H2O=2NaOH+H2��(2��)�����������X���ʿ���Z������ȼ������XZ�������Ϊ��ɫ������ȷ��XΪHԪ�أ�ZΪClԪ�أ�W������Y�����о���ȼ�ղ�����ɫ���棬����W2Y2����ɫ�������������е���ɫ�����WΪNaԪ�أ�YΪOԪ�ء���ÿ2molX2����1molY2��������2molX2Y��X2Y�ڳ�����ΪҺ�壬����X2YΪH2O���ۻ�����XZ��ˮ��Һ��ʹ��ɫʯ����ֽ��죬XZΪHCl����Z�������ڻ�����X2Y��������Һ����Ư�����á�������ˮ��Ӧ���ɵĴ��������Ư���ԡ�

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д�
�����Ŀ