��Ŀ����
����Ŀ����Ҫ��ش�����������
�� ���и������ʣ���O2��O3 ��H2��D2��T2 ��12 C��14 C ��CH3CH2CH2CH3��(CH3)2CHCH3 �ݹ����ʮ���� ��CH3(CH2)5CH3��CH3CH2CH2CH(CH3)C2H5�� (�ں���������Ӧ�����)
(�ں���������Ӧ�����)
A����Ϊͬλ�ص���_____________��B����Ϊͬ���칹�����____________��
C����Ϊͬ�����������____________��D��ͬһ�����ʵ���_____________��
�� д�����������ķ���ʽ
��1������ij��������Է�������Ϊ142����������ķ���ʽΪ ��
��2������A��ͬ��ͬѹ���������ܶ���H2��36�� ��
��3��1L����D��������ȫȼ��ʱ,����ͬ��ͬѹ��15Lˮ���� ��
����1�� ��ϵͳ����������:
�� ,д��������������һȡ����Ӧ�ķ���ʽ
,д��������������һȡ����Ӧ�ķ���ʽ
�� ������һ�ȴ�����в�ͬ�е�IJ����� ��
������һ�ȴ�����в�ͬ�е�IJ����� ��
��2�� д�����и��л���Ľṹ��ʽ��
��2��3��������4���һ����� ��_____________________��
��֧��ֻ��һ���һ�����Է���������С��������___________________��
��3���ǻ��ĵ���ʽ ��
���𰸡�
��A.�� B.�ܢ� C.�� D.��
������1��C10H22 ��2�� C5H12 ��3�� C14H30
����1����2��2������������
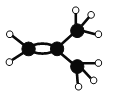
 +Cl2
+Cl2![]() (CH3)3CCH2Cl+HCl ��
(CH3)3CCH2Cl+HCl ��
��3,3,5,5���ļ����飻4��
��2����CH3CH(CH3)CH(CH3)CH(C2H5)CH2CH3��
��CH3CH2CH(C2H5)CH2CH3 ��
��3��![]() ��
��
��������
�������������O2��O3 ��ͬ��Ԫ�����γɵ����ʲ�ͬ�ĵ��ʣ���Ϊͬ�������壻��H2��D2��T2 ������Ԫ����ɵģ�������ͬ�����ʵIJ�ͬ���ӣ���12 C��14 C ��������ͬ����̼Ԫ�صIJ�ͬԭ�ӣ���Ϊͬλ�أ���CH3CH2CH2CH3��(CH3)2CHCH3 ����ʽ��ͬ���ṹ��ͬ��Ϊ̼���칹����Ϊͬ���칹�壻�ݹ����ʮ���� �ṹ���ƣ�������������������������6��CH2ԭ���ţ���CH3(CH2)5CH3��CH3CH2CH2CH(CH3)C2H5 ����ʽ��ͬ���ṹ��ͬ��Ϊ̼���칹����Ϊͬ���칹�壻��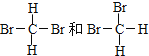 ���Ǽ���Ķ���������ͬ�����ʣ�A����Ϊͬλ�ع�ѡ�ۣ� B����Ϊͬ���칹���ѡ�ܢޣ�C����Ϊͬ���������ѡ�٣�D��ͬһ�����ʹ�ѡ�ߣ��ʴ�Ϊ��A���ۣ�B���ܢޣ�C���٣�D���ߣ�
���Ǽ���Ķ���������ͬ�����ʣ�A����Ϊͬλ�ع�ѡ�ۣ� B����Ϊͬ���칹���ѡ�ܢޣ�C����Ϊͬ���������ѡ�٣�D��ͬһ�����ʹ�ѡ�ߣ��ʴ�Ϊ��A���ۣ�B���ܢޣ�C���٣�D���ߣ�
����1��ij��������Է�������Ϊ142��12n+2n+2=142��n=10������ʽΪC10H22����ʴ�Ϊ��C10H22��
��2������A��ͬ��ͬѹ���������ܶ���H2��36��������������Է�������Ϊ72����12n+2n+2=72��n=5������ʽΪC5H12���ʴ�Ϊ��C5H12��
��3��1L����D��������ȫȼ��ʱ������ͬ��ͬѹ��15Lˮ������˵��1mol������15molˮ����1mol������30molHԭ�ӣ���2n+2=30��n=14������ʽΪC14H30���ʴ�Ϊ��C14H30��
����1���������������������� ������Ϊ2��2���������飬������������һȡ����Ӧ�ķ���ʽΪ
������Ϊ2��2���������飬������������һȡ����Ӧ�ķ���ʽΪ +Cl2
+Cl2![]() (CH3)3CCH2Cl+HCl���ʴ�Ϊ��2��2������������
(CH3)3CCH2Cl+HCl���ʴ�Ϊ��2��2������������ +Cl2
+Cl2![]() (CH3)3CCH2Cl+HCl��
(CH3)3CCH2Cl+HCl��
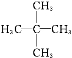
��������������������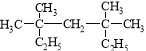 ������Ϊ3,3,5,5���ļ�����������һ�ȴ����ͬ���칹����
������Ϊ3,3,5,5���ļ�����������һ�ȴ����ͬ���칹����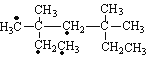 (������ԭ�ӿ��ܵ�λ��)����4�֣��ʴ�Ϊ��3,3,5,5���ļ�������4��
(������ԭ�ӿ��ܵ�λ��)����4�֣��ʴ�Ϊ��3,3,5,5���ļ�������4��
��2���� 2��3��������4���һ�����Ľṹ��ʽΪCH3CH(CH3)CH(CH3)CH(C2H5)CH2CH3���ʴ�Ϊ��CH3CH(CH3)CH(CH3)CH(C2H5)CH2CH3��
��֧��ֻ��һ���һ�����Է���������С��������3-�һ����飬�ṹ��ʽΪCH3CH2CH(C2H5)CH2CH3���ʴ�Ϊ��CH3CH2CH(C2H5)CH2CH3��
��3���ǻ��ĵ���ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��