��Ŀ����
��ҵ�϶Ժ�ˮ��Դ�ۺϿ������õIJ��ֹ�����������ͼ��ʾ��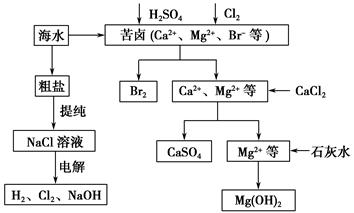
(1)��ⱥ��ʳ��ˮ��������Ĥ���ۺ�Ĥ���ۡ�����Ĥ��Ĥ������ͨ���ķ��ӻ�������________�������е���������Ϊ____________��
(2)�������������Ⱥ��Ƶ�Br2��CaSO4��Mg(OH)2���ܷ�Br2��Mg(OH)2��CaSO4��˳���Ʊ���__________��ԭ����______________________________
(3)�嵥�������Ȼ�̼�е��ܽ�ȱ���ˮ�д�ö࣬���Ȼ�̼��ˮ�����ܣ��ʿ�������ȡ�壬��������������ȴ�������Ȼ�̼��ԭ����________________
(1)������(��Na��)����(��ʯī)
(2)����Ҫ�����ɳ���Mg(OH)2��������л������CaSO4��������Ʒ����
(3)���Ȼ�̼��ȡ�����ո��ӡ��豸Ͷ�ʴ���Ч��͡�������Ⱦ����
����

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д������й���Դ�Ŀ�������˵����ȷ���� �� ��
| A���Ӻ�������ȡ�ⵥ�ʵĹ���һ���漰������ԭ��Ӧ |
| B���Ӻ�ˮ�п��Եõ�NaCl�����NaCl��Һ���Ʊ�Na��Cl2 |
| C����Ȼ�����Ҵ���ˮú���ֱ����ڻ�ʯ��Դ������������Դ�Ͷ�����Դ |
| D��Cu��Al��Hg���Էֱ����Ȼ�ԭCuO�����AlCl3���ȷֽ�HgOұ���õ� |
�ݿ�ѧ��Ԥ�⣺�ٹ�100���ȫ�����¹��ƽ�������Լ1.4��5.8 �档������һԤ�⣬ȫ��������������ȫ���������ɹ����Ӱ�죬����ˮ��Դ���ѷ���������塣��ˮռ�����ܴ�ˮ����97.2%�����Ѻ�ˮ�����ͻ�������������������ܽ����ˮ��Դȱ�������⣬���ܳ�����ú�����Դ��
��1����Ŀǰ�������ԣ������Դ���ĵ����⣬���������ڡ���ˮ�������ļ����� ������ţ���
| A������ | B������������ | C����ᷨ�� | D�����ӽ�������E�������� |
��3����ˮɹ�εõ���ĸҺ�У����д�����þ�������Ӻ�һ�������塢�⻯�������ͨ���Ƚ��ķ��뼼���õ�MgCl2��6H2O��Ʒ���˲�Ʒ����Ҫ�ڲ���ͨ�롰�����Ȼ��⡱����������ˮ���ܵõ���ˮMgCl2��ԭ���� ��
���ʵ���Ժ�ˮ��������Ϊ����ԭ�����Ƶõ����塢�⣬������IJ��������� �� �� ��
��4���ѱ���Ϊ21���ͽ����������ܶ�С��ǿ�ȴ�����һ���ǿ�ᡢǿ����������ܣ��㷺���ں��ա������Լ�����ҽѧ������ҵ�Ͽ�����Mg���»�ԭTiCl4���Ƶá�����ƺ�����ʵ�����������������Ӧ����õ����ѣ������������̣� ��
��������ۺ����ü������ڽ�Լ��Դ���������ڱ���������ʵ�������÷Ͼɻ�ͭ(Cu��Zn�Ͻ𣬺���������Fe)�Ʊ���������(CuSO4��5H2O)��������ZnO���Ʊ�����ͼ���£� 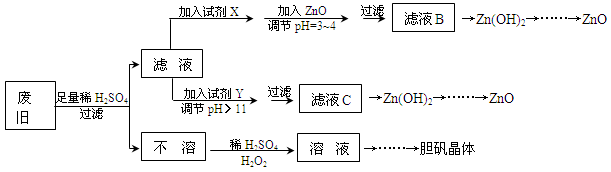
��֪��Zn���������������Al����������������ƣ�pH��11ʱZn(OH)2������NaOH��Һ����[Zn(OH)4]2�����±��г��˼������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0mol��L��1����)��
| | Fe3�� | Fe2�� | Zn2�� |
| ��ʼ������pH | 1.1 | 5.8 | 5.9 |
| ������ȫ��pH | 3.0 | 8.8 | 8.9 |
��ش��������⣺
��1���Լ�X������__________����������____________________��
��2������ZnO����pH=3��4��Ŀ����____________________��
��3���ɲ�����������ҺD�Ļ�ѧ����ʽΪ______________________________��
��4������ҺD�Ƶ��������������Ҫ����������______________________________��
��5�������Լ�����ΪY�Լ�����______��
A��ZnO B��NaOH C��Na2CO3 D��ZnSO4
������ҺC����μ�������ֱ���������������������______________________________��
��6���ⶨ��������Ĵ���(��������I��������Ӧ������������)��ȷ��ȡ0.5000g��������������ƿ�У�������ˮ�ܽ⣬�ټ������KI����0.1000mol��L��1Na2S2O3����Һ�ζ����յ㣬����Na2S2O3����Һ19.40mL����֪�������ζ������е����ӷ���ʽ���£�
2Cu2����4I��
 2CuI(��ɫ)����I2��I2��2S2O32��
2CuI(��ɫ)����I2��I2��2S2O32�� 2I����S4O62��
2I����S4O62���ٵ�������Ĵ���Ϊ_______________��
���ڵζ������о���ҡ��(��Һ���⽦)��ƿ��������õĴ��Ƚ���__________(�ƫ�ߡ�����ƫ�͡����䡱)��
����þ����Ҫ�ɷ�ΪMgCO3������ȼ��������þ�Ĺ����������£�
��1����������ͼ���Եó��Ľ���Ϊ �� ��
ͼ1 25��ʱMgOˮ����ʱ��仯X����������ͼ 
ͼ2 90��ʱMgOˮ����ʱ��仯X����������ͼ
��2��ˮ����ӦMgO+H2O = Mg(OH)2���Է����е�ԭ���� ��
��3�����Ԫ�������ɺͱ�1��֪�����������������ȷֽ�Ĺ����� ����дһ�����ɣ�
��1 ��������Ԫ�صĽ������������ȷֽ��¶�/��
| LiOH | NaOH | KOH | Al(OH)3 | Mg(OH)2 | Ca(OH)2 | Ba(OH)2 |
| 924 | ���ֽ� | ���ֽ� | 140 | 258 | 390 | 700 |
��4����֪�Ȼ�ѧ����ʽ��Mg(OH)2 (s) =" MgO" (s)+H2O (g) ��H =" 81.5" kJ��mol��1
��Mg(OH)2����ȼ���õ���Ҫԭ���� ��
���볣��±ϵ�����������飩���л���ϵ����������������ȼ����ȣ�Mg(OH)2��ȼ�����ŵ��� ��
��ҵ����������������Fe2+��Fe3+�������μ�����CaO��MgO���Ʊ��ߵ���������(Fe2O3 )�ͻ���(NH4)2SO4�����������������£�
�ش��������⣺
��1���ڷ����ܽ����ʱ��Ϊ�˼��ٷ����ܽ�Ĵ�ʩ�ǣ�___________________��__________________����д���㣩
��2������A��һ��������
�ٹ�ҵ�����ѡ�� ������ţ�
| A������ | B��Cl2 | C��MnO2 | D��H2O2�� |
��д��A���뷴Ӧ�����ӷ���ʽΪ__________________________________________
��3������ͼ�й����ݣ�����Ϊ��ҵ����������ʱӦ���Ƶ�������__________________��
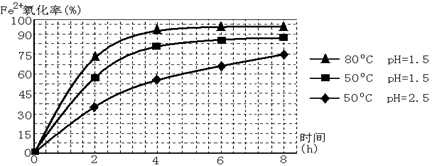
��4����炙�������Һ(��Fe3+)�м�����ҺB��pHΪ5ʱ������������д���������������ӷ���ʽ��_______________________________________________________��
��5��д��炙���������������������ˮ�У������м������Ba(OH)2��Һ��������Ӧ�����ӷ���ʽΪ��_____________________________________________________
��6���������õ�(NH4)2SO4������ܺ��е������ǣ�___________________________
�������ʮ�����Ӻ�ˮ��Դ���ۺ����á����в���Ҫ��ѧ�仯���ܹ��Ӻ�ˮ�л�õ�������( )
| A���ȡ��塢�� | B��ʳ�Ρ���ˮ | C���ռ���� | D���ơ�þ���� |
