��Ŀ����
(12��)ijͬѧΪ��̽��п�����ᷴӦ�����е����ʱ仯������100mLϡ�����м���������п�ۣ����������ˮ�������ռ���Ӧ�ų���������ʵ���¼����(�ۼ�ֵ)��
(1)��һʱ���(ָ0��1��1��2��2��3��3��4��4��5min)��Ӧ�������______________��ԭ����______________________________________________��
(2)��һʱ��εķ�Ӧ������С________________��ԭ����___________________��
(3)��2��3����ʱ����������Ũ�ȱ仯����ʾ�ĸ÷�Ӧ����(����Һ������䣬Ҫд���������)______________________________________________________��
(4)��ͬѧ����ϡ������п��ȡ������ʵ���У����ּ�����������ͭ��Һ�ɼӿ��������������ʡ���ش��������⣺
������ʵ�����漰�������ӷ�Ӧ����ʽ��
��
������ͭ��Һ���Լӿ������������ʵ�ԭ���� ��
��ʵ����������Na2SO4��MgSO4��Ag2SO4��K2SO4��4����Һ������ʵ����CuSO4 ��Һ���������õ��� ��
�ܸ�ͬѧͨ��ʵ���һ���о�������ͭ�����������������ʵ�Ӱ�졣��ͬѧ���ó��Ľ���Ϊ������������CuSO4��Һʱ���������������ʻ�����ߡ����������CuSO4��Һ����һ����ʱ���������������ʷ������½���������������������½�����Ҫԭ��
��
| ʱ��(min) | 1 | 2 | 3 | 4 | 5 |
| �������(mL)(��״��) | 50 | 120 | 232 | 290 | 310 |
(2)��һʱ��εķ�Ӧ������С________________��ԭ����___________________��
(3)��2��3����ʱ����������Ũ�ȱ仯����ʾ�ĸ÷�Ӧ����(����Һ������䣬Ҫд���������)______________________________________________________��
(4)��ͬѧ����ϡ������п��ȡ������ʵ���У����ּ�����������ͭ��Һ�ɼӿ��������������ʡ���ش��������⣺
������ʵ�����漰�������ӷ�Ӧ����ʽ��
��
������ͭ��Һ���Լӿ������������ʵ�ԭ���� ��
��ʵ����������Na2SO4��MgSO4��Ag2SO4��K2SO4��4����Һ������ʵ����CuSO4 ��Һ���������õ��� ��
�ܸ�ͬѧͨ��ʵ���һ���о�������ͭ�����������������ʵ�Ӱ�졣��ͬѧ���ó��Ľ���Ϊ������������CuSO4��Һʱ���������������ʻ�����ߡ����������CuSO4��Һ����һ����ʱ���������������ʷ������½���������������������½�����Ҫԭ��
��
(1) 2��3min��1�֣����¶ȶԷ�Ӧ����Ӱ��ռ�������ã�1�֣���
(2) 4��5min��1�֣���Ũ�ȶԷ�Ӧ����Ӱ��ռ�������ã�1�֣���
(3) 2��3min������V(H2)=" 232" mL -120mL="112" mL=0.112L
n(H2)= =0.005mol
H2SO4 ~ H2��
1 1
n(H2SO4) 0.005mol
����n(H2SO4) =0.005mol
�������ķ�Ӧ����Ϊ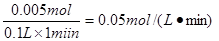 ="0.05" mol/��L?min����2�֣�
="0.05" mol/��L?min����2�֣�
(4)��Zn+Cu2+=Zn2++Cu��1�֣���Zn+2H+=Zn2++H2����1�֣�
�� CuSO4��Zn��Ӧ������Cu��Zn�γ�ԭ��ؼӿ����������������ʡ���1�֣���
�� Ag2SO4��1�֣���
�ܼ���һ������ CuSO4�����ɵĵ���Cu�������Zn�ı��棬������Zn����Һ�ĽӴ������2�֣���
(2) 4��5min��1�֣���Ũ�ȶԷ�Ӧ����Ӱ��ռ�������ã�1�֣���
(3) 2��3min������V(H2)=" 232" mL -120mL="112" mL=0.112L
n(H2)= =0.005mol
H2SO4 ~ H2��
1 1
n(H2SO4) 0.005mol
����n(H2SO4) =0.005mol
�������ķ�Ӧ����Ϊ
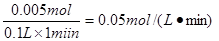 ="0.05" mol/��L?min����2�֣�
="0.05" mol/��L?min����2�֣�(4)��Zn+Cu2+=Zn2++Cu��1�֣���Zn+2H+=Zn2++H2����1�֣�
�� CuSO4��Zn��Ӧ������Cu��Zn�γ�ԭ��ؼӿ����������������ʡ���1�֣���
�� Ag2SO4��1�֣���
�ܼ���һ������ CuSO4�����ɵĵ���Cu�������Zn�ı��棬������Zn����Һ�ĽӴ������2�֣���
��1�����ݱ������ݿ�֪����0��1��1��2��2��3��3��4��4��5min���ռ����������ֱ��ǣ�ml��50��70��112��58��20������2��3min�ڷ�Ӧ��������������ڷ�Ӧ�Ƿ��ȷ�Ӧ���¶ȶԷ�Ӧ���ʵ�Ӱ�쳬����Ũ�ȶԷ�Ӧ���ʵ�Ӱ�졣
��2��4��5min���ռ������������٣����Է�Ӧ������С�������������ŷ�Ӧ�Ľ��У���Ӧ��
��Ũ�ȼ�С����ʱŨ�ȶԷ�Ӧ���ʵ�Ӱ�쳬�����¶ȶԷ�Ӧ���ʵ�Ӱ�졣
��3����
��4����������ͭ���¿����û���ͭ���Ӷ�����ͭпԭ��أ�п���������ӿ췴Ӧ���ʡ���
�����ӷ���ʽΪZn+Cu2+=Zn2++Cu����Zn+2H+=Zn2++H2�����������Ľ�����Ҳ����п�ģ���
��Ҳ�ܺ�п����ԭ��أ����������ͭ���Ƶ������������������̫ͭ�࣬�����ɵĵ���Cu
�������Zn�ı��棬������Zn����Һ�ĽӴ�������Ӷ����ͷ�Ӧ���ʡ�
��2��4��5min���ռ������������٣����Է�Ӧ������С�������������ŷ�Ӧ�Ľ��У���Ӧ��
��Ũ�ȼ�С����ʱŨ�ȶԷ�Ӧ���ʵ�Ӱ�쳬�����¶ȶԷ�Ӧ���ʵ�Ӱ�졣
��3����
��4����������ͭ���¿����û���ͭ���Ӷ�����ͭпԭ��أ�п���������ӿ췴Ӧ���ʡ���
�����ӷ���ʽΪZn+Cu2+=Zn2++Cu����Zn+2H+=Zn2++H2�����������Ľ�����Ҳ����п�ģ���
��Ҳ�ܺ�п����ԭ��أ����������ͭ���Ƶ������������������̫ͭ�࣬�����ɵĵ���Cu
�������Zn�ı��棬������Zn����Һ�ĽӴ�������Ӷ����ͷ�Ӧ���ʡ�

��ϰ��ϵ�д�
 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
�����Ŀ
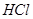 �����������
�����������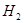 ���������������أ�������Ũ�Ȣ��¶Ȣ�þ���ı�������������ܱ��������Һ��
���������������أ�������Ũ�Ȣ��¶Ȣ�þ���ı�������������ܱ��������Һ��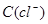 ��С���й�ϵ���ǣ� ��
��С���й�ϵ���ǣ� ��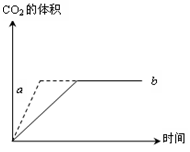
 �����ʵ����������õ������ǹ����ģ�����ȡ��״ʯ��ʯ���ĩ״ʯ��ʯ�������Ƿ���ȣ������ȡ�����ȡ�����
�����ʵ����������õ������ǹ����ģ�����ȡ��״ʯ��ʯ���ĩ״ʯ��ʯ�������Ƿ���ȣ������ȡ�����ȡ�����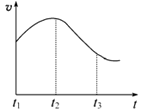
 2SO3
2SO3