��Ŀ����
����Ŀ������(H2C2O4)��һ�ֶ�Ԫ���ᡣ��������H2C2O4��Һ�еμ�NaOH��Һ�������Һ��lgX[X��ʾ![]() ��
��![]() ]��pH�ı仯��ϵ��ͼ��ʾ������˵������ȷ����
]��pH�ı仯��ϵ��ͼ��ʾ������˵������ȷ����
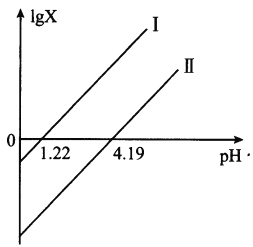
A. ֱ��I��X��ʾ����![]()
B. ֱ��I������б�ʾ�Ϊ1
C. c(HC2O4��)>c(C2O42��)>c(H2C2O4)��Ӧ1.22<pH<4.19
D. c(Na+)=c(HC2O4��)+2c(C2O42��)��ӦpH=7
���𰸡�C
��������
A������lgX=0ʱ��pH=-lgc(H��)=-lgK,�����жϣ�
B����pH=0������㣻
C������ƽ�ⳣ������ʽ�����ͼ��lgX=0ʱ�������pH��
D��Ӧ�õ���غ�����
A����Ԫ��������K1=c(H��)c(HC2O4��)/c(H2C2O4)>K2=c(H��)c(C2O42��)/c(HC2O4��)����lgX=0ʱ��pH=-lgc(H��)=-lgK,pH1=1.22<pH2=4.19������K1=10-1.22>K2=10-4.19������ ֱ��I��X��ʾ����c(HC2O4��)/c(H2C2O4)��ֱ�ߢ���X��ʾ����c(C2O42��)/c(HC2O4��)����A��ȷ��
B��pH=0ʱ��lgc(HC2O4��)/c(H2C2O4)=lgK1=-1.22��lgc(C2O42��)/c(HC2O4��)=lgK2=-4.19�����ԣ�ֱ��I��II��б�ʾ�Ϊ1����B��ȷ��
C����pH=a,c(H��)=10-a,c(C2O42��)/c(HC2O4��)=K2/c(H��)=10a-4.19����c(C2O42��)<c(HC2O4��)ʱ��10a-4.19<1����a-4.19<0����ã�a<4.19��K1��K2=c(H��)c(HC2O4��)/c(H2C2O4)��c(H��)c(C2O42��)/c(HC2O4��)=c2(H��)c(C2O42��)/c(H2C2O4)����c(C2O42��)/c(H2C2O4)=K1��K2/c2(H��)=102a-5.41����c(C2O42��)>c(H2C2O4)ʱ��102a-5.41>1��2a-5.41>0�����a>2.705������c(HC2O4��)>c(C2O42��)>c(H2C2O4)��Ӧ2.705<pH<4.19����C����
D������غ㣺 c(Na+)+c(H��)=c(HC2O4��)+2c(C2O42��)+c(OH��)����c(Na+)=c(HC2O4��)+2c(C2O42��)ʱ��c(H��)=c(OH��)����ӦpH=7����D��ȷ��
��ѡC��

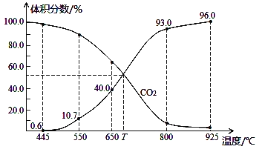
����Ŀ����2L�ܱ������ڣ�800��ʱ��Ӧ��2NO(g)+O2(g)![]() 2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
ʱ��(s) | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)(mol) | 0.020 | 0.01. | 0.008 | 0.007 | 0.007 | 0.007 |
��1����֪��K300����K350����д���÷�Ӧ��ƽ�ⳣ������ʽ��K=_________________�����ڸ÷�Ӧ������˵���У���ȷ����________��
A����H>0����S>0 B����H>0����S<0
C����H<0����S<0 D����H<0����S>0
��2����ͼ�б�ʾNO2�ı仯��������____________________����O2��ʾ��0-2s�ڸ÷�Ӧ��ƽ������v=_______________��
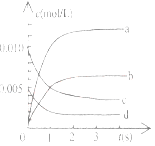
��3����˵���÷�Ӧ�Ѵﵽƽ��״̬����__________��
A��v(NO2)=2v(O2) B��������ѹǿ���ֲ���
C��v (NO)=2v��O2�� D���������ܶȱ��ֲ���
��4�����д�ʩ����ʹn(NO2)/n(NO)�������____��(����ĸ)
A�������¶� B���������
C�����ϳ���O2 D������He(g)��ʹ��ϵ��ѹǿ����