��Ŀ����
���и�������ʾ��ͼһ�µ��ǣ�������


������ͼ���漰�������ӷ���ʽ�У�Mg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3����NH4++OH-�TNH3?H2O��
Al��OH��3+OH-�TAlO2-+2H2O����NaOH��Һ��Ũ��Ϊxmol/L����n��NH4+��=0.01xmol��n��Al��OH��3��=n��Al3+��=0.005xmol��
n��Mg2+��=
=0.005xmol����n��Mg2+����n��Al3+����n��NH4+��=1��1��2��
����������þ�����ʵ�����֪��0.005xmol=0.05mol��x=10mol/L��
ͼ�������߱�ʾ��Ӧ���������������Ĵ�С����A�Ƿ�Ӧ���Ӧ���ȣ���֮���ȣ�B��Ϊ��ܣ������������ܽ��ͣ�
Al��OH��3+OH-�TAlO2-+2H2O����NaOH��Һ��Ũ��Ϊxmol/L����n��NH4+��=0.01xmol��n��Al��OH��3��=n��Al3+��=0.005xmol��
n��Mg2+��=
| 0.025xmol-0.005��3xmol |
| 2 |
����������þ�����ʵ�����֪��0.005xmol=0.05mol��x=10mol/L��
ͼ�������߱�ʾ��Ӧ���������������Ĵ�С����A�Ƿ�Ӧ���Ӧ���ȣ���֮���ȣ�B��Ϊ��ܣ������������ܽ��ͣ�
����⣺A��ͼ���漰�������ӷ���ʽ�У�Mg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3����NH4++OH-�TNH3?H2O��
Al��OH��3+OH-�TAlO2-+2H2O����NaOH��Һ��Ũ��Ϊxmol/L����n��NH4+��=0.01xmol��n��Al��OH��3��=n��Al3+��=0.005xmol��
nn��Mg2+��=
=0.005xmol����n��Mg2+����n��Al3+����n��NH4+��=1��1��2����A����
B����NaOH��Һ��Ũ��Ϊxmol/L������n��Mg2+��=n��Mg��OH��2��=0.005xmol=0.05mol��x=10mol/L����B����
C��ͼ���б�ʾij��Ӧ���̵������仯��ʹ�ô������ͷ�Ӧ�Ļ�ܣ��ӿ췴Ӧ���ʣ���C��ȷ��
D��ͼ�������߱�ʾ��Ӧ���������������Ĵ�С����A�Ƿ�Ӧ���Ӧ���ȣ���H��0����D����
��ѡC��
Al��OH��3+OH-�TAlO2-+2H2O����NaOH��Һ��Ũ��Ϊxmol/L����n��NH4+��=0.01xmol��n��Al��OH��3��=n��Al3+��=0.005xmol��
nn��Mg2+��=
| 0.025xmol-0.005��3xmol |
| 2 |
B����NaOH��Һ��Ũ��Ϊxmol/L������n��Mg2+��=n��Mg��OH��2��=0.005xmol=0.05mol��x=10mol/L����B����
C��ͼ���б�ʾij��Ӧ���̵������仯��ʹ�ô������ͷ�Ӧ�Ļ�ܣ��ӿ췴Ӧ���ʣ���C��ȷ��
D��ͼ�������߱�ʾ��Ӧ���������������Ĵ�С����A�Ƿ�Ӧ���Ӧ���ȣ���H��0����D����
��ѡC��
���������⿼������Ũ�ȵļ����Լ���Ӧ�����仯��֪ʶ�������Է�Ӧ��Ӱ�죬�����ѶȽϴ�ע�����ͼ������㡢�յ㡢�յ㡢�仯���Ƶ�����ķ�����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
���и�������ʾ��ͼһ�µ��ǣ�������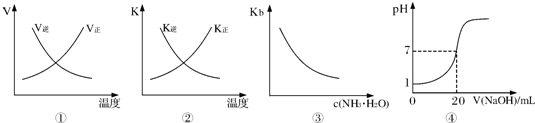

| A��ͼ�ٱ�ʾ��ӦN2��g��+O2��g��?2NO��g����H��0�����淴Ӧ���������¶ȵı仯 | B��ͼ�ڱ�ʾ��ӦN2��g��+3H2��g��?2NH3��g����H��0�����淴Ӧ��ƽ�ⳣ�����¶ȵı仯 | C��ͼ�۱�ʾ��ˮ�ĵ���ƽ�ⳣ���氱ˮŨ��c��NH3?H2O���ı仯 | D��ͼ�ܱ�ʾ25��ʱ����0.1mol?L-1NaOH��Һ�ζ�20mL 0��lmol?L-1���ᣬ��Һ��pH����������ı仯 |
����ʱ�����и�������ʾ��ͼһ�µ��ǣ�������
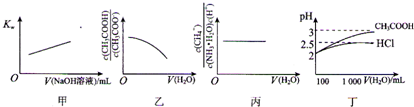

| A��ͼ�ױ�ʾ�ڴ�ˮ�м���0.01 moL?L-1 NaOH��Һ��Kw��NaOH��Һ����仯��ϵ | ||
B��ͼ�ұ�ʾ��1 mol?L-1 CH3COONa��Һ��ˮϡ�ͣ���Һ��
| ||
C��ͼ����ʾ0.1mol?L-1 NH3?H2O��ˮϡ�ͣ���Һ��
| ||
| D��ͼ����ʾ100 mL pH=2��HCl��CH3COOH��ˮϡ�͵�1000mLʱpH�仯��ˮ������ı仯��ϵ |

