ЬтФПФкШн
ЁОЬтФПЁПЂё.ЃЈ1ЃЉ25Ёц 101 kPaЪБЃЌЧтЦјКЭбѕЦјЗДгІЩњГЩ1molЫЎеєЦјЗХШШ241.8kJЃЌИУЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊ____ЁЃ
ЃЈ2ЃЉШє1gЫЎеєЦјзЊЛЏЮЊвКЬЌЫЎЗХШШ2.444 kJЃЌдђЗДгІ2H2(g)ЃЋO2(g)=2H2O(l)ЕФІЄHЃН___ЃЌгЩДЫПЩжЊЧтЦјЕФШМЩеШШЮЊ____ЁЃ(НсЙћБЃСєаЁЪ§ЕуКѓвЛЮЛ)
Ђђ.вбжЊHЃЋ(aq)ЃЋOHЃ(aq)=H2O(l) ІЄHЃНЃ57.3kJ/molЃЌЛиД№ЯТСагаЙижаКЭЗДгІЕФЮЪЬтЃК
ЃЈ1ЃЉгУ0.1molBa(OH)2ХфГЩЯЁШмвКгызуСПЯЁЯѕЫсЗДгІЃЌФмЗХГі___kJЕФФмСПЁЃ
ЃЈ2ЃЉШчЭМЫљЪОзАжУжаЃЌвЧЦїAЕФУћГЦЪЧ___ЃЌзїгУЪЧ__ЃЛЫщХнФЫмСЯЕФзїгУЪЧ___ЁЃ
ЃЈ3ЃЉЭЈЙ§ЪЕбщВтЖЈЕФжаКЭШШЕФІЄHГЃГЃДѓгкЃ57.3kJ/molЃЌЦфдвђПЩФмЪЧ___ЁЃ
ЃЈ4ЃЉгУЯрЭЌХЈЖШКЭЬхЛ§ЕФАБЫЎ(NH3ЁЄH2O)ДњЬцNaOHШмвКНјааЩЯЪіЪЕбщЃЌВтЕУЕФжаКЭШШЕФЪ§жЕ____(ЬюЁАЦЋДѓЁБЁЂЁАЦЋаЁЁБЛђЁАЮогАЯьЁБ)ЁЃ
ЁОД№АИЁПH2(g)ЃЋ![]() O2(g)=H2O(g) ІЄHЃНЃ241.8 kJ/mol Ѓ571.6kJ/mol 285.8kJ/mol 11.46 ЛЗаЮВЃСЇНСАшАє НСАшЃЌЪЙШмвКГфЗжЛьКЯ БЃЮТЁЂИєШШЁЂМѕЩйЪЕбщЙ§ГЬжаЕФШШСПЫ№ЪЇ ЪЕбщжаВЛПЩБмУтгаЩйСПШШСПЫ№ЪЇ ЦЋаЁ
O2(g)=H2O(g) ІЄHЃНЃ241.8 kJ/mol Ѓ571.6kJ/mol 285.8kJ/mol 11.46 ЛЗаЮВЃСЇНСАшАє НСАшЃЌЪЙШмвКГфЗжЛьКЯ БЃЮТЁЂИєШШЁЂМѕЩйЪЕбщЙ§ГЬжаЕФШШСПЫ№ЪЇ ЪЕбщжаВЛПЩБмУтгаЩйСПШШСПЫ№ЪЇ ЦЋаЁ
ЁОНтЮіЁП
Ђё.(1)ИљОнЧтЦјКЭбѕЦјЗДгІЩњГЩ1molЫЎеєЦјЗХШШ241.8kJЃЌЪщаДЗДгІЕФШШЛЏбЇЗДгІЗНГЬЪНЃЛ
(2)ИљОн1gЫЎеєЦјзЊЛЏГЩвКЬЌЫЎЗХШШ2.444kJЃЌМЦЫу1molЫЎеєЦјзЊЛЏЮЊвКЬЌЫЎЗХГіЕФШШСПЃЌдйИљОнИЧЫЙЖЈТЩМЦЫуЦфьЪБфЃЛ
Ђђ. (1)гЩH+(aq)+OH-(aq)ЈTH2O(l)ЁїH=-57.3kJmol-1ПЩжЊЩњГЩ1molH2OЗХГіШШСПЮЊ57.3kJЃЌШЛКѓИљОнЫЎЕФЮяжЪЕФСПгыШШСПГЩе§БШЧѓГіШШСПЃЛ
(2)ИљОнЭМЪОХаЖЯвЧЦїУћГЦЃЛжаКЭШШВтЖЈЪБгІОЁСПМѕЩйШШСПЩЂЪЇЃЛ
(3)ЪЕМЪЪЕбщЙ§ГЬжавЛЖЈгаШШСПЩЂЪЇЃЛ
(4)NH3H2OЮЊШѕЕчНтжЪЃЌЕчРыашвЊЮќШШЃЌОнДЫЗжЮіХаЖЯЁЃ
Ђё.(1)гЩЧтЦјКЭбѕЦјЗДгІЩњГЩ1molЫЎеєЦјЗХШШ241.8kJЃЌЩњГЩ2molЫЎеєЦјЗХГіШШСПЮЊ483.6kJЃЌИУЗДгІШШЛЏбЇЗДгІЗНГЬЪНЮЊH2(g)ЃЋ![]() O2(g)=H2O(g) ІЄHЃНЃ241.8 kJ/molЛђ2H2(g)+O2(g)=2H2O(g)ЁїH=-483.6kJ/molЃЌЙЪД№АИЮЊЃКH2(g)ЃЋ
O2(g)=H2O(g) ІЄHЃНЃ241.8 kJ/molЛђ2H2(g)+O2(g)=2H2O(g)ЁїH=-483.6kJ/molЃЌЙЪД№АИЮЊЃКH2(g)ЃЋ![]() O2(g)=H2O(g) ІЄHЃНЃ241.8 kJ/molЛђ2H2(g)+O2(g)=2H2O(g)ЁїH=-483.6kJ/molЃЛ
O2(g)=H2O(g) ІЄHЃНЃ241.8 kJ/molЛђ2H2(g)+O2(g)=2H2O(g)ЁїH=-483.6kJ/molЃЛ
(2)Шє1gЫЎеєЦјзЊЛЏГЩвКЬЌЫЎЗХШШ2.444kJЃЌдђ1molЫЎеєЦјзЊЛЏЮЊвКЬЌЫЎЗХГіШШСП= =43.992kJ/molЃЌИљОнИЧЫЙЖЈТЩжЊЦфьЪБф=-483.6kJ/mol+(-43.992kJ/mol)ЁС2=-571.58kJ/molЁж-571.6kJ/molЃЌдђЧтЦјЕФШМЩеШШ=
=43.992kJ/molЃЌИљОнИЧЫЙЖЈТЩжЊЦфьЪБф=-483.6kJ/mol+(-43.992kJ/mol)ЁС2=-571.58kJ/molЁж-571.6kJ/molЃЌдђЧтЦјЕФШМЩеШШ=![]() =285.8kJ/molЃЌЙЪД№АИЮЊЃК-571.6kJ/molЃЛ285.8kJ/molЃЛ
=285.8kJ/molЃЌЙЪД№АИЮЊЃК-571.6kJ/molЃЛ285.8kJ/molЃЛ
Ђђ.(1)гЩH+(aq)+OH-(aq)ЈTH2O(l)ЁїH=-57.3kJmol-1ПЩжЊЩњГЩ1molH2OЗХГіШШСПЮЊ57.3kJЃЌ0.1mol Ba(OH)2ХфГЩЯЁШмвКгызуСПЯЁЯѕЫсЗДгІПЩЕУ0.2molH2OЃЌЫљвдЗХГіЕФШШСПЮЊ57.3kJЁС0.2=11.46kJЃЌЙЪД№АИЮЊЃК11.46ЃЛ
(2)ИљОнЭМЪОЃЌвЧЦїAЪЧЛЗаЮВЃСЇНСАшАєЃЌПЩЦ№ЕННСАшЃЌЪЙШмвКГфЗжЛьКЯЕФФПЕФЃЛжаКЭШШВтЖЈЪЕбщГЩАмЕФЙиМќЪЧБЃЮТЙЄзїЃЌДѓаЁЩеБжЎМфЬюТњЫщХнФЫмСЯПЩвдЦ№ЕНБЃЮТЁЂИєШШЃЌМѕЩйШШСПЩЂЪЇЕФзїгУЃЌЙЪД№АИЮЊЃКЛЗаЮВЃСЇНСАшАєЃЛНСАшЃЌЪЙШмвКГфЗжЛьКЯЃЛБЃЮТЁЂИєШШЃЌМѕЩйЪЕбщЙ§ГЬжаЕФШШСПЩЂЪЇЃЛ
(3) ЪЕМЪЪЕбщЙ§ГЬжавЛЖЈгаВПЗжШШСПЩЂЪЇЃЌвђДЫЧѓЕУЕФжаКЭШШЁїHДѓгк-57.3kJmol-1ЃЌЙЪД№АИЮЊЃКЪЕбщЙ§ГЬжаВЛПЩБмУтгаШШСПЩЂЪЇЃЛ
(4)NH3H2OЮЊШѕМюЃЌЕчРыЙ§ГЬЮЊЮќШШЙ§ГЬЃЌгУЯрЭЌХЈЖШКЭЬхЛ§ЕФАБЫЎ(NH3H2O)ДњЬцNaOHШмвКНјааЩЯЪіЪЕбщЃЌВтЕУЕФжаКЭШШЕФЪ§жЕЦЋаЁЃЌЙЪД№АИЮЊЃКЦЋаЁЁЃ

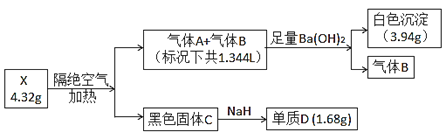
 ОйвЛЗДШ§ЕЅдЊЭЌВНЙ§ЙиОэЯЕСаД№АИ
ОйвЛЗДШ§ЕЅдЊЭЌВНЙ§ЙиОэЯЕСаД№АИЁОЬтФПЁПЯћГ§ЮВЦјжаЕФNOЪЧЛЗОГПЦбЇбаОПЕФШШЕуПЮЬтЁЃ
IЃЎNOбѕЛЏЛњРэ
вбжЊЃК2NO(g)+O2(g) ![]() 2NO2(g)ЁїH=-110kJЁЄmol-1
2NO2(g)ЁїH=-110kJЁЄmol-1
25ЁцЪБЃЌНЋNOКЭO2АДЮяжЪЕФСПжЎБШЮЊ2:1ГфШыКуШнЗДгІШнЦїжаЃЌгУВтбЙЗЈбаОПЦфЗДгІЕФНјааЧщПіЁЃЬхЯЕЕФзмбЙЧПpЫцЪБМфtЕФБфЛЏШчЯТБэЫљЪО(КіТдNO2гыN2O4ЕФзЊЛЏ)
t/min | 0 | 80 | 160 |
|
p/kPa | 75.0 | 63.0 | 55.0 | 55.0 |
ЃЈ1ЃЉ0~80minЃЌv(O2)=_____kPa/minЃЛЫцзХЗДгІНјааЃЌЗДгІЫйТЪж№НЅМѕаЁЕФдвђЪЧ______________ЁЃ
гУЦНКтЗжбЙДњЬцЦНКтХЈЖШЫљЕУЕНЕФЦНКтГЃЪ§гУK(p)БэЪОЃЌ25ЁцЪБЃЌK(p)ЕФжЕЮЊ_______(БЃСє3ЮЛгааЇЪ§зж)ЁЃ
ЃЈ2ЃЉВщдФзЪСЯЃЌЖдгкзмЗДгІ2NOg)+O2(g) ![]() 2NO2(g)гаШчЯТСНВНРњГЬ
2NO2(g)гаШчЯТСНВНРњГЬ
ЕквЛВН2NO(g) ![]() N2O2(g) ПьЫйЗДгІ
N2O2(g) ПьЫйЗДгІ
ЕкЖўВНN2O2(g)+O2(g) ![]() 2NO2(g) Т§ЗДгІ
2NO2(g) Т§ЗДгІ
змЗДгІЫйТЪжївЊгЩЕк_____ВНОіЖЈЃЛШєРћгУЗжзгВЖЛёЦїЪЪЕБМѕЩйЗДгІШнЦїжаЕФN2O2ЃЌзмЗДгІЕФЦНКтГЃЪ§K(p)НЋ___(ЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБ)ЃЛШєЬсИпЗДгІЮТЖШжС35ЁцЃЌдђЬхЯЕбЙЧПP(35Ёц)______P(25Ёц)(ЬюЁАДѓгкЁБЁЂЁАЕШгкЁБЛђЁАаЁгкЁБ)ЁЃ
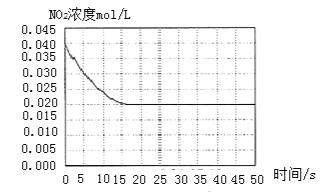
II. ФГЮТЖШЯТвЛУмБеШнЦїжаГфШывЛЖЈСПЕФNO2ЃЌВтЕУNO2ХЈЖШЫцЪБМфБфЛЏЕФЧњЯпШчЩЯЭМЫљЪОЁЃ
ЃЈ1ЃЉЗДгІЬхЯЕДяЦНКтКѓбЙЧПЮЊP1ЃЌШєЩ§ИпЮТЖШЃЌдйДЮДяЦНКтКѓЃЌЛьКЯЦјЬхЕФЦНОљЯрЖдЗжзгжЪСП______![]() ЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБ
ЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБ![]() ЃЛ
ЃЛ
ЃЈ2ЃЉШєдкКуЮТКуШнЬѕМўЯТЃЌЯђЦНКтЬхЯЕжаГфШывЛЖЈСПO2ЃЌдйДЮДяЦНКтКѓЃЌВтЕУбЙЧПЮЊP2ЃЌc(O2)=0.09mol/LЃЌдђP2ЃКP1=______
ЃЈ3ЃЉИУЮТЖШЯТЗДгІ2NO(g)+O2(g) ![]() 2NO2(g)ЕФЛЏбЇЦНКтГЃЪ§KЮЊ______ЁЃ
2NO2(g)ЕФЛЏбЇЦНКтГЃЪ§KЮЊ______ЁЃ