��Ŀ����
��10�֣�Fe�����岻��ȱ�ٵ���Ԫ�أ����뺬��������ɲ������������������壨FeSO4��7H2O����ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�
��1������ڼ������H2O2��Ŀ�ģ� ��
��2��������з�Ӧ�����ӷ���ʽ�� ��
��3���������һϵ�д����IJ������裺 ��ϴ�ӡ� ����ȴ��������
��4��ʵ������Ũ��������1 mol/�̵�ϡ���ᣬ����ʱ�õ��Ķ������������� �� ��
��5����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص����� g���ú�a�Ĵ���ʽ��ʾ����
��1����Fe2+ȫ������ΪFe3+
��2��Fe3++3NH3��H2O=Fe(OH)3��+3NH4+
��3������ ����
(4) ����ƿ ��Ͳ
��5��0.07a
����:��1����Һ�к����������ӣ�����˫��ˮ���Խ����������������ӣ������ڳ�����
��2�����κͼӦ�Ϳ���������������������
��3����������������ˮ������ͨ�����˵õ����壬�����˳���������������Ҫͨ��ϴ�ӣ�Ȼ�����ռ�ת��Ϊ��������
��4��Ũ��������ϡ������Ҫ��Ͳ������ƿ�����ֶ�������������
��5��ag�������к�����Ԫ�ص�������![]() ������ÿƬ�к��е�̼Ԫ�ص�������
������ÿƬ�к��е�̼Ԫ�ص�������![]() ��
��

 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д�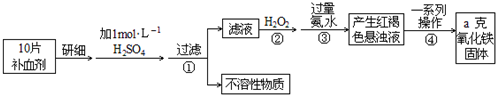
 6SO2��Fe3O4������3 mol FeS2�μӷ�Ӧ����ת�� mol���ӡ�
6SO2��Fe3O4������3 mol FeS2�μӷ�Ӧ����ת�� mol���ӡ�