��Ŀ����
16����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ�D������ˮ�γɵ���Һ����Ư���ԣ�E��ԭ������Ϊ24��ED3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磮���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ����1��A��B��C�ĵ�һ��������С�����˳��ΪC��O��N��
��2����֪A��D�γɵĻ�������ӿռ乹��Ϊ�������壬��������COCl2�����ӿռ乹��Ϊƽ�������Σ�˵��ԭ��CCl4����ԭ�Ӳ�ȡsp3�ӻ�����COCl2����ԭ����sp2�ӻ���������COCl2��������̼��ԭ�Ӽ�Ĺ��ۼ��ļ�����D������ţ���
A��1���Ҽ� B��2�Ҹ��� C��2���м� D��1���Ҽ���1���м�
��3��д�������ʵ�����D���ʺ�SO2����ͬʱͨ��ˮ�з�Ӧ�����ӷ���ʽSO2+Cl2+2H2O=4H++2Cl-+SO42-��
��4��ED3��B��C���⻯���γɵ������Ļ�ѧʽΪ[Cr��NH3��4��H2O��2]Cl3��
���� A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��D������ˮ�γɵ���Һ����Ư���ԣ���DΪCl��A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�ء�CΪOԪ�أ�Bԭ����������̼����֮�䣬��BΪNԪ�أ�E��ԭ������Ϊ24����EΪCr��CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3���ݴ˽��
��� �⣺A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��D������ˮ�γɵ���Һ����Ư���ԣ���DΪCl��A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�ء�CΪOԪ�أ�Bԭ����������̼����֮�䣬��BΪNԪ�أ�E��ԭ������Ϊ24����EΪCr��CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
��1��AΪ̼Ԫ�ء�BΪ��Ԫ�ء�CΪ��Ԫ�أ�ͬ����������ҵ�һ����������Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�����������ͣ���Ԫ�ص�һ�����ܸ������ڵ�Ԫ�صģ����Ե�һ��������С�����˳��ΪC��O��N��
�ʴ�Ϊ��C��O��N��
��2��A��D�γɵĻ��������ΪCCl4��������COCl2�����ӵĽṹʽΪ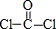 ��CCl4����ԭ�Ӳ�ȡsp3�ӻ�����COCl2����ԭ����sp2�ӻ�����CCl4Ϊ��������ṹ��COCl2Ϊƽ�������Σ�������COCl2��������̼��ԭ�Ӽ�Ĺ��ۼ��ļ�����1���Ҽ���1���м���
��CCl4����ԭ�Ӳ�ȡsp3�ӻ�����COCl2����ԭ����sp2�ӻ�����CCl4Ϊ��������ṹ��COCl2Ϊƽ�������Σ�������COCl2��������̼��ԭ�Ӽ�Ĺ��ۼ��ļ�����1���Ҽ���1���м���
�ʴ�Ϊ��CCl4����ԭ�Ӳ�ȡsp3�ӻ�����COCl2����ԭ����sp2�ӻ���D��
��3�������ʵ�����������SO2����ͬʱͨ��ˮ������HCl�����ᣬ��Ӧ�����ӷ���ʽΪ��SO2+Cl2+2H2O=4H++2Cl-+SO42-��
�ʴ�Ϊ��SO2+Cl2+2H2O=4H++2Cl-+SO42-��
��4��CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3���ʴ�Ϊ��[Cr��NH3��4��H2O��2]Cl3��
���� ���������ʽṹ���ۺ�����Ŀ���漰�ṹ����Խλ�ù�ϵ��Ԫ�������ɡ���������ӻ����ۡ����ӽṹ�ȣ��Ѷ��еȣ��Ƕ�ѧ���ۺ������Ŀ��飮

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�| A�� | ��SO2Ư������ | |
| B�� | ����ˮ��ͨ��������������ɱ�� | |
| C�� | ��ʳ����ϴ��ˮƿ���ڱڸ��ŵ�ˮ����ˮ������Ҫ�ɷ�ΪCaCO3��Mg��OH��2�� | |
| D�� | ��С�մ�NaHCO3���������������ͷ |
| A�� | ��Ϊs�������״�����εģ�����s����������Բ���˶� | |
| B�� | 3px��3py��3pz�IJ���֮�����������е��ӣ���̬����������ͬ | |
| C�� | ԭ�ӹ���͵����ƶ���������������������˶�״̬�� | |
| D�� | ������ͼ�ϵ�ÿһ���㶼����һ������ |
| A�� | ���ӻ������п��ܺ��й��ۼ�������һ�����н���Ԫ�� | |
| B�� | ������һ�����й��ۼ� | |
| C�� | �Ǽ��Է�����һ�����ڷǼ��Լ� | |
| D�� | ���Է�����һ�������ڷǼ��Լ� |
| A�� | п��������ͭ�Ǹ��� | B�� | ���Ӵ�ͭƬ����������пƬ | ||
| C�� | �����ķ�ӦʽΪ2H++2e-=H2�� | D�� | ��Ӧһ��ʱ�����Һ��pH���� |
| A�� | CH3CH2Br+CH3COONa��CH3COOCH2CH3+NaBr | |
| B�� | CH3I+CH3ONa��CH3OCH3+NaI | |
| C�� | CH3CH2Cl+CH3ONa��CH3Cl+CH3CH2ONa | |
| D�� | CH3CH2Cl+CH3CH2ONa����CH3CH2�� 2O+NaCl |

 ��F
��F ��
��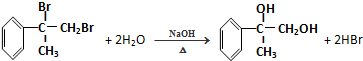 ��
�� ��
��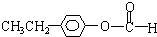 ��
��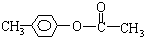 ��
��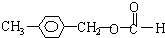 ��
��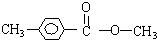 ��
��