��Ŀ����
ij�о���ѧϰС��������Ʒ�������������ƣ�
��1�������Ǹ�С��ͬѧ�����ʵ�����Ʊ������ļ��ַ�����
A.����粒������������ƹ��干��
B.���ȷֽ�NH4Cl����
C.��Ũ��ˮ��μӵ����Ƶ���ʯ����
����Ϊ���н�Ϊ������еķ�����_______________����д��ĸ�����䷴Ӧ�Ļ�ѧ����ʽΪ__________________________________��
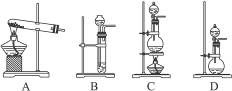
�����ͼ��������ѡ����ȡ�����ķ���װ�ã�Ҫ�������١�������__________��д��ţ���
��2����С�����ռ������İ�����
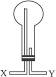
����ѡ������ͼ��ʾ��װ�ã�������Ӧ�ɵ��ܿ�_______���X����Y�������루����ƿ���ܵߵ�����
��������Һ�������ռ���������ѡ�õ��Լ���_______������ĸ��
A.H2O B.ŨH2SO4 C.CCl4 D.NaCl������Һ
(3)��ͼʾװ�ý�����Ȫʵ�飬�ϲ���ƿ��װ�����ﰱ��������ˮ����IJ�����___________
________________________��
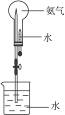
��4����ԭ��ƿ�а����dz����ģ�������Ȫʵ�����ƿ����Һ�����ʵ����ʵ���Ũ��Ϊ_________���ٶ��ڱ�״���£���
��1�������Ǹ�С��ͬѧ�����ʵ�����Ʊ������ļ��ַ�����
A.����粒������������ƹ��干��
B.���ȷֽ�NH4Cl����
C.��Ũ��ˮ��μӵ����Ƶ���ʯ����
����Ϊ���н�Ϊ������еķ�����_______________����д��ĸ�����䷴Ӧ�Ļ�ѧ����ʽΪ__________________________________��

�����ͼ��������ѡ����ȡ�����ķ���װ�ã�Ҫ�������١�������__________��д��ţ���
��2����С�����ռ������İ�����

����ѡ������ͼ��ʾ��װ�ã�������Ӧ�ɵ��ܿ�_______���X����Y�������루����ƿ���ܵߵ�����
��������Һ�������ռ���������ѡ�õ��Լ���_______������ĸ��
A.H2O B.ŨH2SO4 C.CCl4 D.NaCl������Һ
(3)��ͼʾװ�ý�����Ȫʵ�飬�ϲ���ƿ��װ�����ﰱ��������ˮ����IJ�����___________
________________________��

��4����ԭ��ƿ�а����dz����ģ�������Ȫʵ�����ƿ����Һ�����ʵ����ʵ���Ũ��Ϊ_________���ٶ��ڱ�״���£���
��1��C CaO+NH3��H2O====Ca(OH)2+NH3�� D
(2)��X ��C ��3����ֹˮ�У�������ͷ�ι��е�ˮ
��4��0.045 mol��L-1
(2)��X ��C ��3����ֹˮ�У�������ͷ�ι��е�ˮ
��4��0.045 mol��L-1
��1����������ȷֽ⣬�¶Ȳ�ͬ���ﲻͬ��������������ը���Ȼ�立ֽ���ȻҲ�������������������Ȼ����������Թܿ����ܻ��ϳ��Ȼ�泥���ˣ�ֻ�ܽ���ˮ��μӵ����Ƶ���ʯ���С���2�������ܶȱȿ���С�����������ſ������ռ���������������ˮ����������ˮ��������Ũ�������백����Ӧ��Ҳ�������������ռ�����4������ƿ�����ΪV L����c(NH3��H2O)=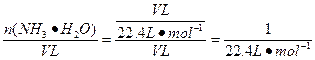 ="0.045" mol��L-1��
="0.045" mol��L-1��
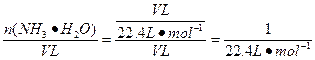 ="0.045" mol��L-1��
="0.045" mol��L-1��
��ϰ��ϵ�д�
�����Ŀ

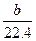 mol
mol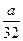 mol
mol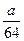 mol
mol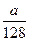 mol
mol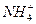 ��H3O+��
��H3O+��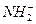 ��OH-��N3-��O2-�������ƣ��ݴ��ж����з�Ӧ����ȷ���ǣ� ��
��OH-��N3-��O2-�������ƣ��ݴ��ж����з�Ӧ����ȷ���ǣ� �� Mg3N2+3NH3��
Mg3N2+3NH3��