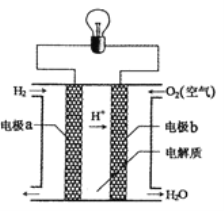
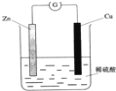
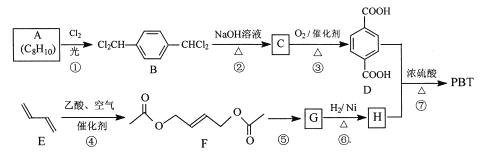
��Ŀ����
����Ŀ��ij��ѧ��ȤС���ȡ������Na2SO3 ��7H20 a�ˣ�����������ǿ�������أ�������������㣬�õ��Ĺ���������ȫ��ת��Ϊ�������ƹ���ļ���ֵһ�£���������ˮ���ܽ����pHֵ�����ۼ���ֵ����ͬŨ��Na2SO3��Һ��pH����ܶࡣ����˵������ȷ����
A. �������γ���ǿ��ǰ��������������������С��0.1g�������жϹ����Ѿ�����
B. ��������ì�ܵĿ��ܽ��ͣ�4Na2SO3 ![]() 3Na2SO4 �� Na2S
3Na2SO4 �� Na2S
C. ��������м���ϡ��������е���ɫ��������
D. ����BaCl2��Һ�����ְ�ɫ����������ȷ����������Na2SO4
���𰸡�D
��������A���������γ���ǿ��ǰ��������������������С��0.1g�������жϹ����Ѿ����أ���A��ȷ��B������Na2SO3�Ⱦ����������־��л�ԭ�ԣ�Na2SO3���Ⱥ������绯��Ӧ����Ԫ�ػ��ϼ۴�+4�۱仯Ϊ-2�ۺ�+6�ۣ���Ӧ�Ļ�ѧ����ʽΪ��4Na2SO3=Na2S+3Na2SO4����B��ȷ��C�������п��ܻ���Na2SO3��Na2SO3��Na2S�����������¿��Թ��з�Ӧ�������ʣ����Թ�������м���ϡ��������е���ɫ������������C��ȷ��D�������п��ܻ���Na2SO3������BaCl2��Һ�����ְ�ɫ����������ȷ����������Na2SO4����D����ѡD��